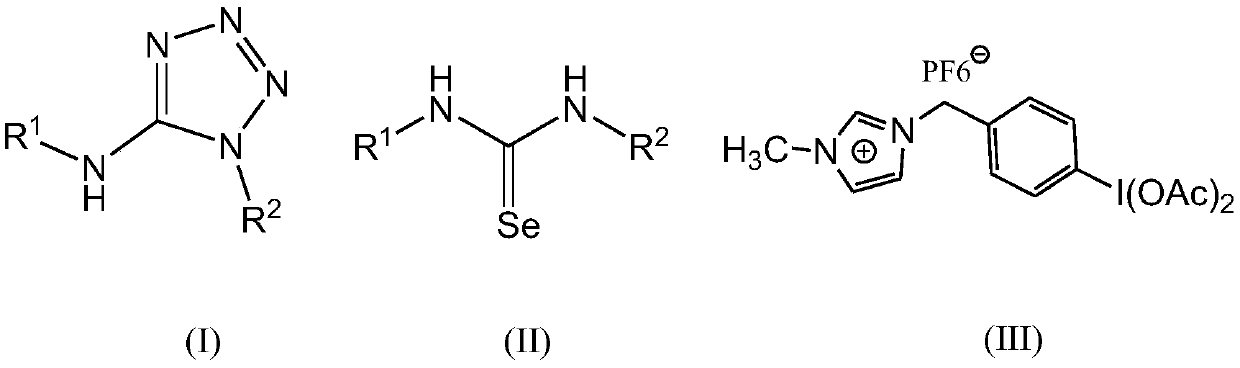
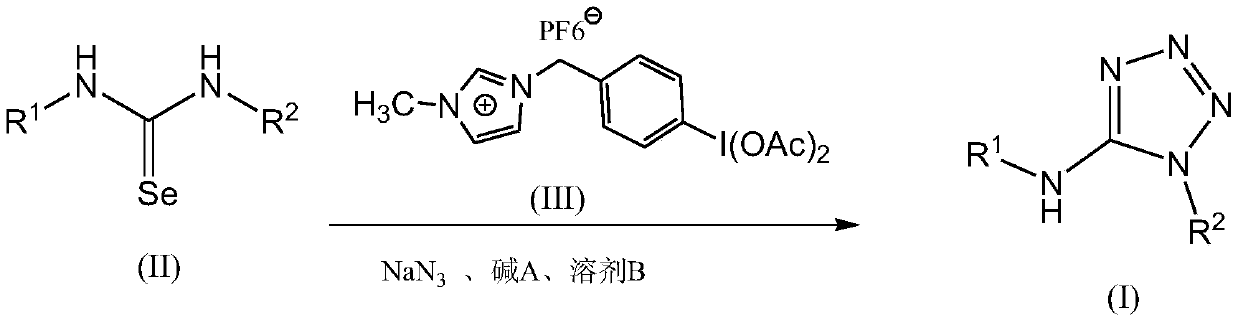
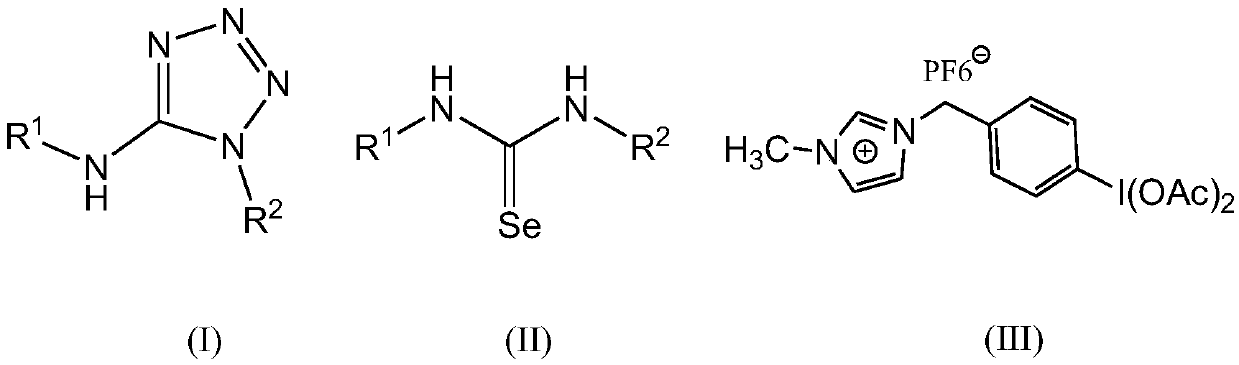
Method for preparing 5-aminotetrazole compound
A technology for aminotetrazoles and compounds, which is applied in the field of preparation of 5-aminotetrazoles, can solve the problems of using heavy metal reagents, harsh reaction conditions, and long reaction time, so as to avoid the use of heavy metals and mild reaction conditions , The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Add 1,3-diphenylselenourea (275mg, 1mmol), ionic liquid-loaded iodobenzene diacetate (674mg, 1.2mmol) and sodium azide (195mg, 3mmol) into a 50mL single-necked bottle, add DMF (10mL ) and H 2 O (1 mL), NaOH (80 mg, 2 mmol) was added, and stirred at 25° C. for 0.2 h. After the TLC monitoring reaction finishes, filter, pour the filtrate into saturated NaHCO 3 In the aqueous solution, the layers were allowed to stand, and the obtained aqueous layer was extracted with ethyl acetate, and the combined organic layers were washed with anhydrous Na 2 SO 4 After drying and concentration, it was separated by column chromatography (eluent petroleum ether: ethyl acetate volume ratio = 4:1). The eluate containing the product was collected, and the solvent was evaporated to obtain the white target product Ia with a yield of 0.225 g and a yield of 95% (document 76%: New Journal of Chemistry, 2013, 37(2), 488-493).
[0042] Melting point: 158-160°C (literature 158-159°C)...
Embodiment 2
[0044]
[0045] Add 1,3-di-o-ethylphenylselenourea (331mg, 1mmol), ionic liquid-loaded iodobenzene diacetate (450mg, 0.8mmol) and sodium azide (260mg, 4mmol) into a 50mL single-necked bottle, add Dimethylsulfoxide (15mL) and H 2 O (2mL), then add Cs 2 CO 3 (326mg, 1mmol), stirred at 50°C for 1h. After the TLC monitoring reaction finishes, filter, pour the filtrate into saturated NaHCO 3 In the aqueous solution, the layers were allowed to stand, and the obtained aqueous layer was extracted with dichloromethane, and the combined organic layers were washed with anhydrous Na 2 SO 4 After drying and concentration, it was separated by column chromatography (eluent petroleum ether: ethyl acetate volume ratio = 4:1). The eluate containing the product was collected, and the solvent was distilled off to obtain the white target product Ib with a yield of 0.152 g and a yield of 52%.
[0046] Melting point: 109-111°C; 1 HNMR (400MHz, CDCl 3 )δ8.10(d,J=8.0Hz,1H),7.58(td,J 1 =7.6...
Embodiment 3
[0048]
[0049] Add 1,3-di-p-chlorophenylselenourea (344mg, 1mmol), ionic liquid-loaded iodobenzene diacetate (843mg, 1.5mmol) and sodium azide (130mg, 2mmol) into a 50mL single-necked bottle, add toluene (30mL) and H 2 O (0.1 mL), then added KOH (140 mg, 2.5 mmol), stirred at 80° C. for 0.1 h. After the TLC monitoring reaction finishes, filter, pour the filtrate into saturated NaHCO 3 In the aqueous solution, the layers were allowed to stand, and the obtained aqueous layer was extracted with ethyl acetate, and the combined organic layers were washed with anhydrous Na 2 SO 4 After drying and concentration, it was separated by column chromatography (eluent petroleum ether: ethyl acetate volume ratio = 4:1). The eluate containing the product was collected, and the solvent was distilled off to obtain the white target product Ic, with a yield of 0.217 g and a yield of 71%.
[0050] Melting point: 194-195°C; IR(KBr)ν max 3271, 2920, 1610, 1570, 1491, 1410, 1090, 820; 1 HNM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com