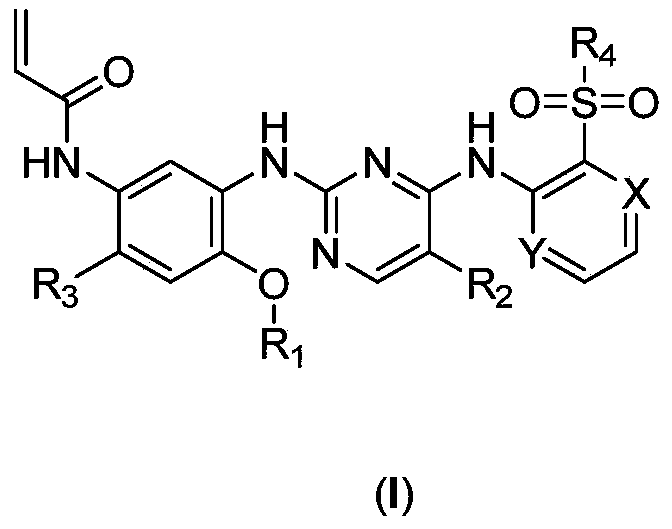
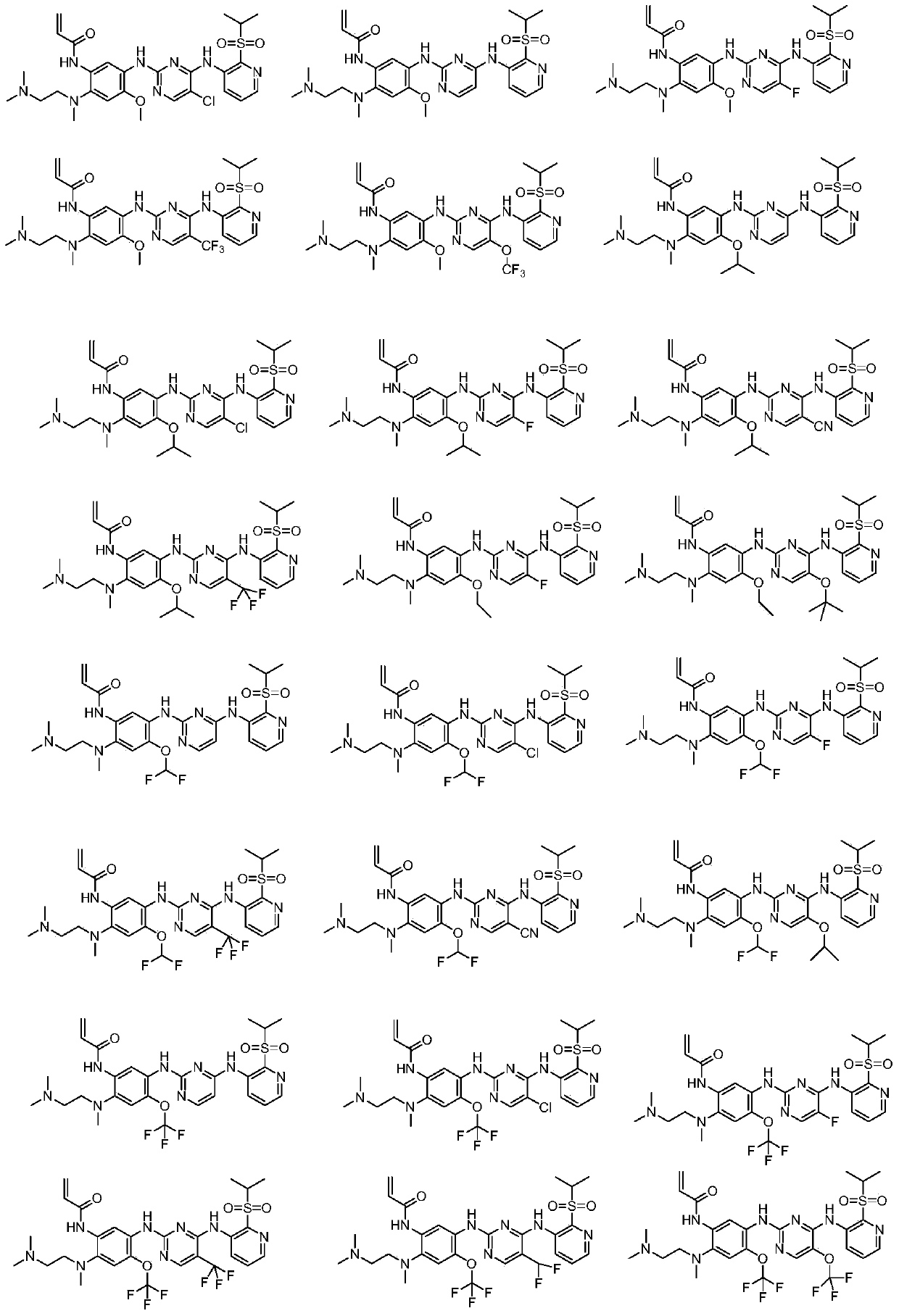
EGFR (Epidermal growth factor receptor) inhibitor and preparation method and use thereof
An alkyl and amino technology, applied in the field of drug synthesis, can solve problems such as poor selectivity, limited dosage, and reduced affinity and drug resistance between drugs and targets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] Preparation of intermediates
[0069] 1. Intermediate 1: Preparation of 4-fluoro-2-methoxy-5-nitroaniline
[0070]
[0071] 4-Fluoro-2-methoxynitrobenzene (10 g, 58.44 mmol) was dissolved in methanol, 1 g of palladium / carbon was added, replaced by hydrogen, and reacted under hydrogen atmosphere for three days. Filtrate, collect the filtrate, and distill methanol off to obtain an oily crude product. Concentrated sulfuric acid was added to the crude product at 0°C to dissolve all the solids in the mixture, and potassium nitrate (5.91 g) was added in batches. The reaction solution was slowly warmed to room temperature and stirred overnight. The reaction solution was poured into ice water, NaHCO was added 3 , adjust pH6.0-8.0. The aqueous phase was extracted three times with dichloromethane, and the organic phase was collected and dried. The organic solvent was evaporated, and the residue was separated and purified by column chromatography to obtain 4-fluoro-2-meth...
Embodiment 1
[0096] Embodiment 1: N-(5-((5-chloro-4-((2-(isopropylsulfonyl)pyridin-3-yl)amino)pyrimidin-2-yl)amino)-2-((2 Preparation of -(dimethylamino)ethyl)(methyl)amino)-4-methoxyphenyl)acrylamide
[0097]
[0098] The first step: the preparation of 2-isopropylmercapto-3-nitropyridine
[0099]
[0100] Put 2-fluoro-3-nitropyridine (500mg, 3.52mmol), potassium carbonate (973mg, 7.04mmol) in a 100mL round bottom bottle, add 15ml of DMF, add isopropylthiol (0.36mL, 3.87mmol) under stirring ), the mixture was reacted at room temperature for 1 hour, and the reaction solvent was rotated off after the reaction was completed. The crude product obtained was washed with water, extracted with ethyl acetate, dried and concentrated, and then column chromatography to obtain the product 2-isopropylmercapto-3-nitro Pyridine (660 mg, 95% yield).
[0101] LC-MS: t=4.31min, 198.9 (M+H + );
[0102] 1 HNMR (400MHz, CDCl 3 )δ8.61(dd, J=4.8, 1.6Hz, 1H), 8.40(dd, J=8.4, 1.6Hz, 1H), 7.10(dd, J=8.4...
Embodiment 2
[0131] Example 2: N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-5-((4-((2-(isopropylsulfonyl)pyridin-3-yl )amino)pyrimidin-2-yl)amino)-4-methoxyphenyl)acrylamide preparation
[0132]
[0133] N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-5-((4-((2-(isopropylsulfonyl)pyridin-3-yl)amino)pyrimidine -2-yl)amino)-4-methoxyphenyl)acryloylamide The preparation method is the same as the fourth to eighth steps of Example 1.
[0134] LC-MS: t R =2.02min, 569.3([M+H] + );
[0135] 1 HNMR (400MHz, CDCl 3 )δ9.81(s,2H), 9.01(m,2H), 8.57(d,1H), 8.02(s,1H), 7.24-7.35(m,3H), 6.79(s,1H), 6.10(m ,2H), 5.48(d,1H), 3.88(s,3H), 3.80(m,1H), 2.93(br,2H), 2.73(s,3H), 2.36(m,8H), 1.43(d, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com