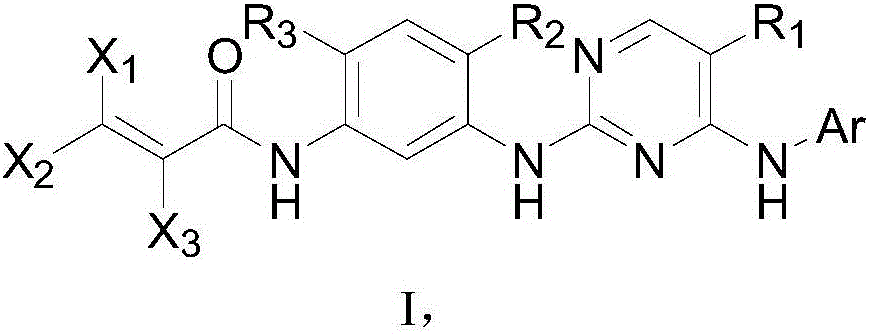
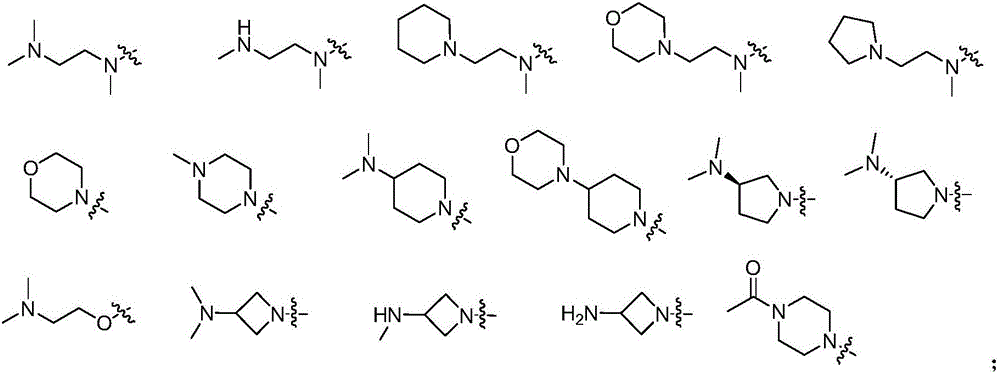
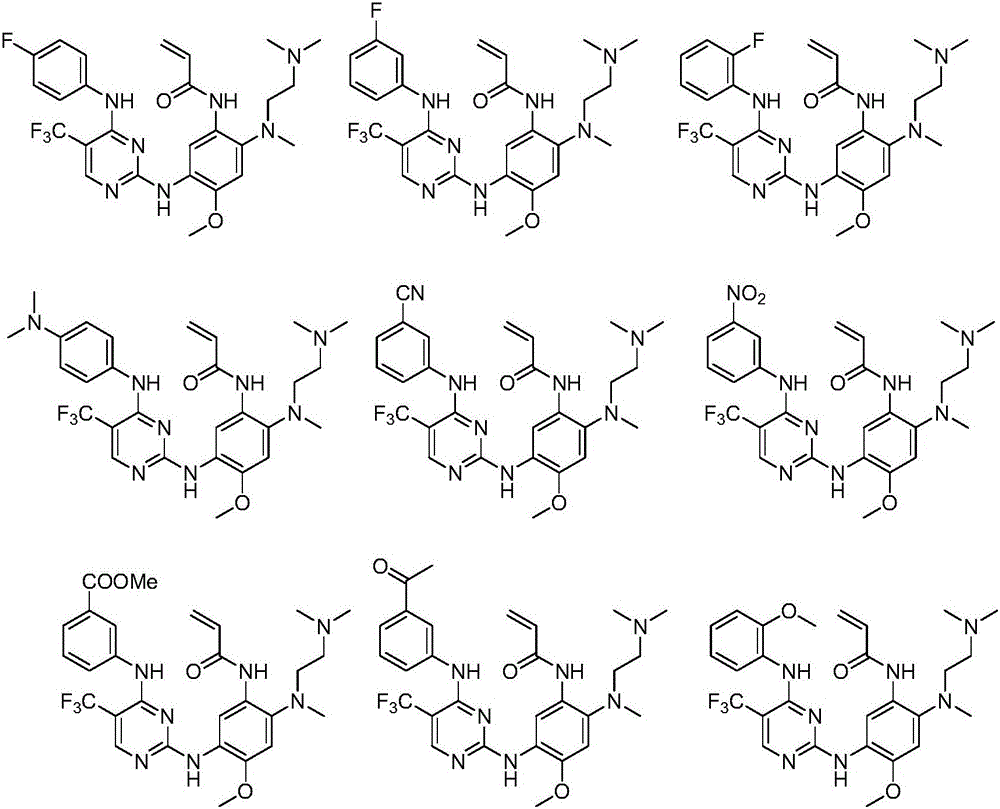
Pyrimidine compound, EGFR inhibitor and application of EGFR inhibitor
A compound and pyrimidine technology, applied in the field of medicine, can solve the problems of severe rash and side effects in patients, poor efficacy in drug-resistant patients, etc., and achieve the effects of low toxicity, reduced inhibitory activity, and reduced mutation selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] The preparation method of intermediate A is method A1, method A2 or method A3, specifically as follows.
[0073] Method A1: Add 7.8mmol of compound a1-1 to a 100ml single-necked bottle, add 3ml of diisopropylethylamine and 30ml of n-butanol to obtain a mixture; use a cold bath to cool the mixture to -20°C, and slowly Add compound a2 (13.8mmol) dropwise, react at low temperature for 1h after the addition, remove the cooling bath and warm up to room temperature, stir overnight, evaporate the solvent to dryness under reduced pressure, add 100ml of ethyl acetate (EA) to the residue, and then add 50ml washed with water twice, the organic phase was evaporated to dryness, and the residue was separated by column chromatography (eluent: ethyl acetate:petroleum ether=1:30 (volume ratio)) to obtain intermediate A1.
[0074] The reaction formula that above-mentioned method A1 involves is as follows:
[0075]
[0076] Wherein, Ar is selected from phenyl or substituted phenyl, an...
Embodiment 1
[0117] The pyrimidine compound of the present embodiment has a structural formula as shown in formula I-1:
[0118]
[0119] The preparation method of the pyrimidine compound of the present embodiment is as follows: 50mg (0.15mmol) of intermediate B, 150mg (0.5mmol) of intermediate A and 35mg (0.18mmol) of p-toluenesulfonic acid monohydrate are dissolved in 5ml of 2 -amyl alcohol, then warming up to 50°C, stirring overnight under nitrogen protection, TLC showed that the raw material basically disappeared, spin to dry volume, then add 20ml dichloromethane and 20ml saturated aqueous sodium carbonate solution, separate layers, then wash with 20ml dichloromethane The aqueous phase was collected twice, and the organic phase was combined, dried and spin-dried, and separated by TLC to obtain 20 mg of the product, which was compound I-1. The analytical data of this compound are as follows: 1H-NMR (400MHz, CDCl 3 ): δ: 10.18 (br, 1H); 9.19 (br, 1H); 8.38 (s, 1H); 7.52 (dt, J = 2.39...
Embodiment 30
[0135] The pyrimidine compound of the present embodiment is the mesylate of the pyrimidine compound (I-6) shown in Example 6, and its structural formula is as shown in formula I-30:
[0136]
[0137] The preparation method of the pyrimidine compound (methanesulfonate) of this example is: add 0.37g of compound I-6 into a 50ml one-mouth bottle, add 10ml of acetone and 1ml of water, and slowly add it under stirring after the addition After adding 64mg of methanesulfonic acid, react at 50°C for 3h, evaporate the reaction solution to dryness, add 6ml of acetonitrile and heat up to 70°C, stir for 30min, cool slowly to precipitate the solid, filter the solid, wash with acetonitrile, After drying, 140 mg of a white solid was obtained, namely compound I-30. Its purity was 98.5% as detected by HPLC. The analytical data of gained compound 1-30 is: HNMR (400M, d 6 -DMSO): 9.35(s1H), 9.14(s,1H), 8.76(s,1H), 8.70(s,1H), 8.36(s,1H), 7.95(s,2H), 7.85(d,J= 8.10Hz, 1H), 7.41(m, 2H), 6.97(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com