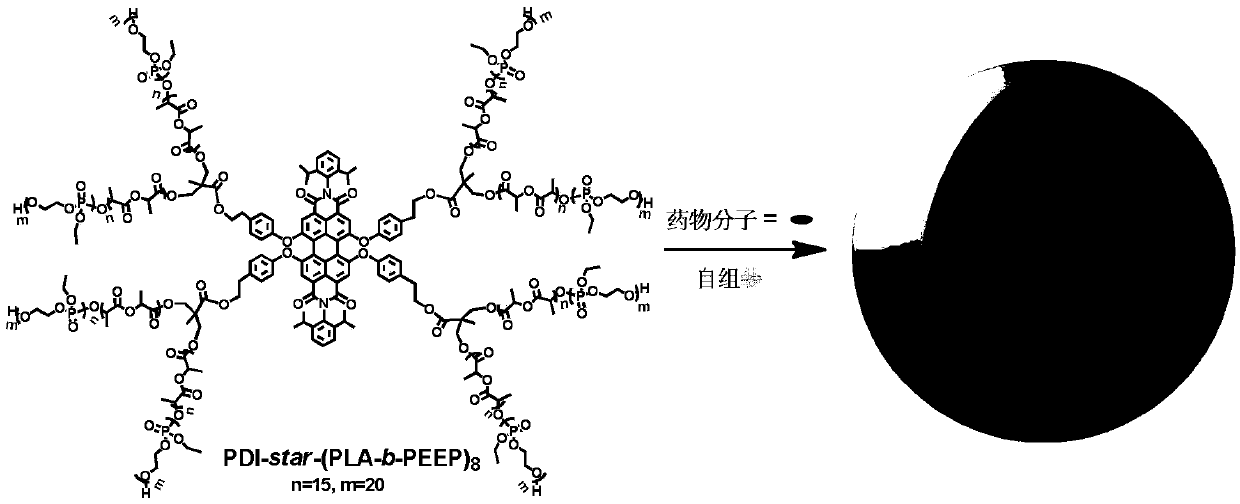
Preparation and application of fluorescent star-shaped block copolymer
A technology of block copolymers and copolymers, which can be used in preparations for in vivo tests, medical preparations with non-active ingredients, and pharmaceutical formulas, etc., which can solve the problem of peryleneimide's easy aggregation, weak fluorescence properties, and limited applications, etc. problem, to achieve the effect of good optical performance, good fluorescence characteristics, excellent biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
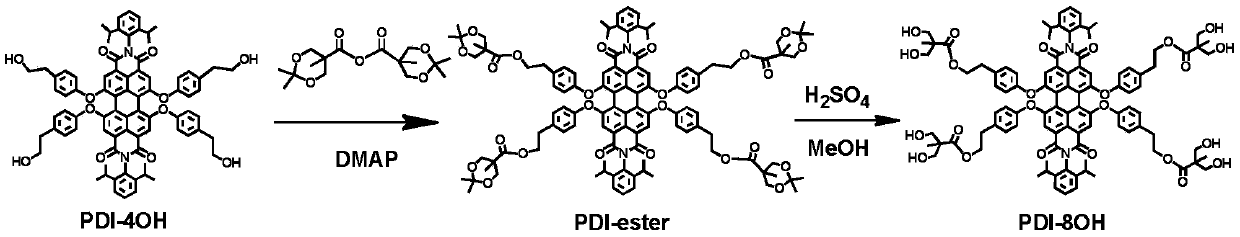
[0038] (1) Synthesis of eight-arm initiator compound 2: as attached figure 1 As shown, compound 5 (100mg, 0.08mmol), 4-dimethylaminopyridine (42mg, 0.32mmol) and triethylamine (0.23mL, 1.6mmol) were added to 16mL of tetrahydrofuran, under nitrogen protection and 0°C conditions, A 10 mL tetrahydrofuran solution in which compound 6 (1.06 g, 3.2 mmol) was dissolved was added dropwise. After the dropwise addition, it was stirred at room temperature (25°C) for 24 hours. After the reaction, the tetrahydrofuran was removed by a rotary evaporator, the resulting mixture was dissolved in 20 mL of dichloromethane, and then saturated NaHSO 4 Wash with aqueous solution (3×50 mL), saturated NaCl aqueous solution (3×50 mL) and deionized water (3×50 mL). The organic phase was purified with a silica gel column to obtain 135 mg of intermediate product, which was designated as PDI-ester, and the yield was 90%. The intermediate product PDI-ester (100mg, 0.053mmol) was dissolved in 20mL methanol, ...
Embodiment 2
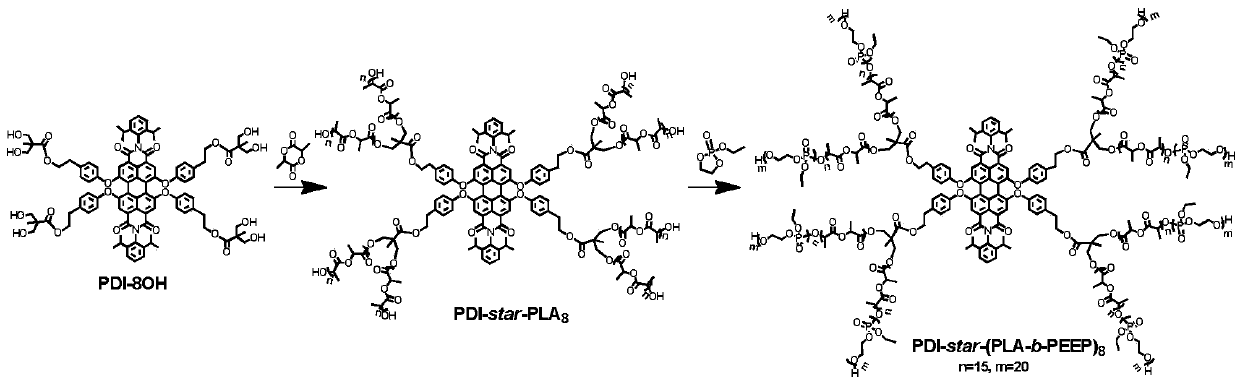
[0051] (1) PDI-star-PLA prepared in step 2) of Example 1 8 (100mg, 0.0053mmol) and monomer 4 (R1 is ) (400 mg, 1.50 mmol) was added to 0.4 mL of dichloromethane to dissolve it, and 3 freeze-pump cycles were performed. Under the protection of nitrogen, the catalyst 1,8-diazabicyclo[5.4.0]undec-7-ene (0.05 mL) was added, and after freezing and pumping again, the reaction was carried out at 35°C for 10 hours. After the reaction, it was precipitated in ether (3×50 mL), and the solid obtained was dried to constant weight under vacuum to obtain a copolymer, which was recorded as PDI-star-(PLA 13 -b-PPEEABoc 18 ) 8 , The yield is 56.7%. PDI-star-(PLA 13 -b-PEEABoc 18 ) 8 (200mg) dissolved in 0.6mL of dichloromethane, under nitrogen protection and room temperature, add 0.6mL of trifluoroacetic acid dropwise, continue to stir for 3 hours; after the completion of the reaction, remove the trifluoroacetic acid with a rotary evaporator, and precipitate in ether (3×50mL), the obtained solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com