Method for producing sterilized medical molded body
A manufacturing method and molding technology, which can be applied in the directions of disinfection and irradiation, and can solve the problems of not recording the types and amounts of antioxidants.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
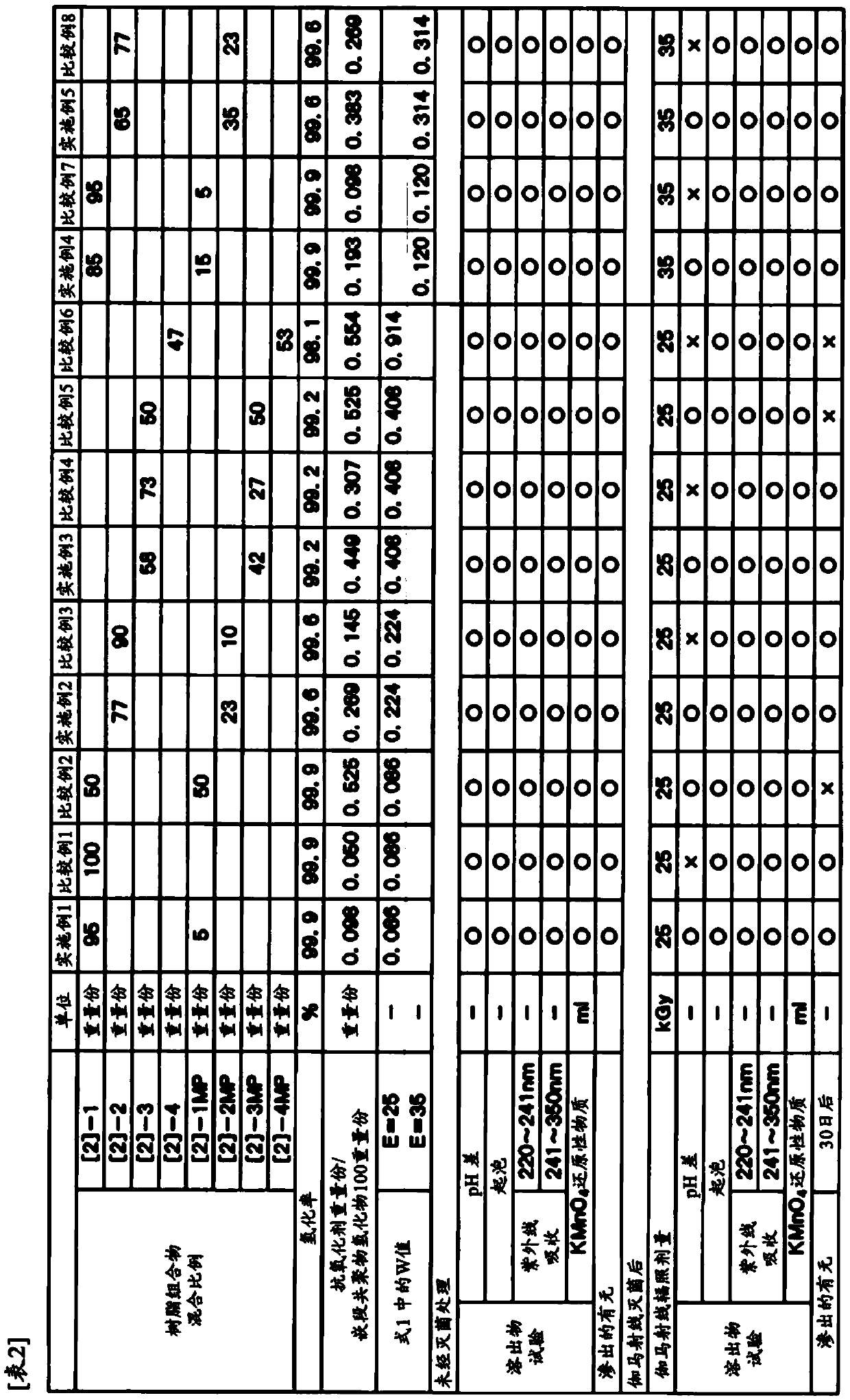
[0081] Hereinafter, the present invention will be described in more detail with reference to examples and comparative examples, but the present invention is not limited to these examples. In the following examples and comparative examples, parts and % are based on weight unless otherwise specified.
[0082] The measurement methods of various physical properties are shown below.
[0083] (1) Weight average molecular weight (Mw) and molecular weight distribution (Mw / Mn)
[0084] The molecular weight of the block copolymer and the hydrogenated product of the block copolymer was measured at 38°C as a value in terms of standard polystyrene by gel permeation chromatography (GPC) using THF as an eluent. As a measuring device, HLC8020GPC manufactured by Tosoh Corporation was used.
[0085] (2) Hydrogenation rate
[0086] The hydrogenation rate of the hydrogenated block copolymer is the proportion of hydrogenated carbon-carbon bonds relative to the total amount of carbon-carbon unsa...
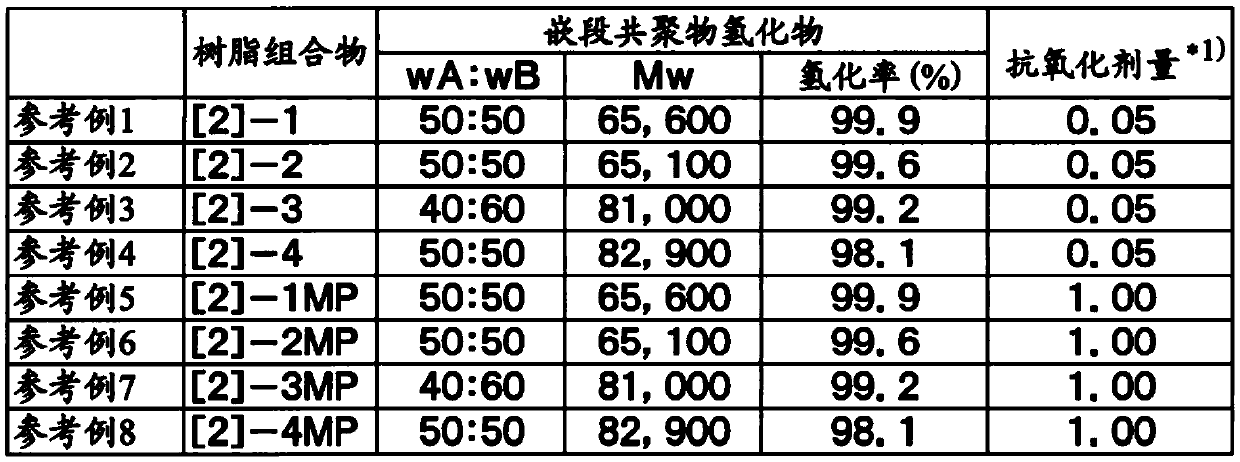
reference example 1
[0101] Synthesis of Resin Composition [2]-1 Consisting of Hydrogenated Block Copolymer and Antioxidant
[0102] 550 parts of dehydrated cyclohexane, 25.0 parts of dehydrated styrene, and 0.475 parts of di-n-butyl ether were charged into a reactor equipped with a stirring device whose interior was sufficiently replaced with nitrogen. While stirring the entire contents at 60°C, 0.67 parts of n-butyllithium (15% cyclohexane solution) was added to initiate polymerization, and the reaction was further carried out for 60 minutes while stirring at 60°C. As a result of measuring the reaction solution by gas chromatography, the polymerization conversion rate at this point was 99.5%.
[0103] Then, 50.0 parts of dehydrated isoprene was added to the reaction liquid, and stirring was continued at 60° C. for 30 minutes while maintaining the state. As a result of measuring the reaction solution by gas chromatography, the polymerization conversion rate was 99% at this point.
[0104] Then,...
reference example 2
[0112] Synthesis of Resin Composition [2]-2 Consisting of Hydrogenated Block Copolymer and Antioxidant
[0113] Except for using 3.5 parts of the hydrogenation catalyst, pellets 94 of a resin composition ([2]-2) composed of a hydrogenated block copolymer ([1]-2) and an antioxidant were obtained in the same manner as in Reference Example 1. share.
[0114]The hydrogenated block copolymer of the obtained resin composition ([2]-2) had a weight average molecular weight (Mw) of 65,100, a molecular weight distribution (Mw / Mn) of 1.11, a hydrogenation rate of 99.6%, and wA:wB=50: 50. The amount of the antioxidant is 0.05 parts by weight relative to 100 parts by weight of the hydrogenated block copolymer ([1]-2). These data are described in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com