Perovskite type compound preparation method
A perovskite type and compound technology, applied in the direction of tin halide, etc., can solve the problems of harsh process conditions, expensive raw materials, and impure products, and achieve the effects of simple synthesis process, simple process reduction, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
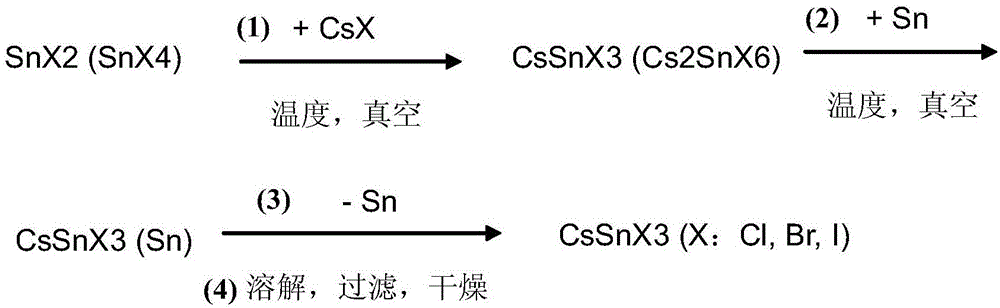
[0049] The preparation method of perovskite type compound, specifically the method for preparing tin cesium trihalide, its technological process is as follows figure 1 As shown, the specific steps are as follows:
[0050] Preparation of High Purity CsSnI3 Solid
[0051] 1. Synthesis of CsSnI3 (Cs2SnI6):
[0052] 1) Reaction formula:
[0053] SnI2+CsI---------CsSnI3
[0054] SnI4 (a small amount)+2CsI--------Cs2SnI6 (a small amount)
[0055] 2) specific method
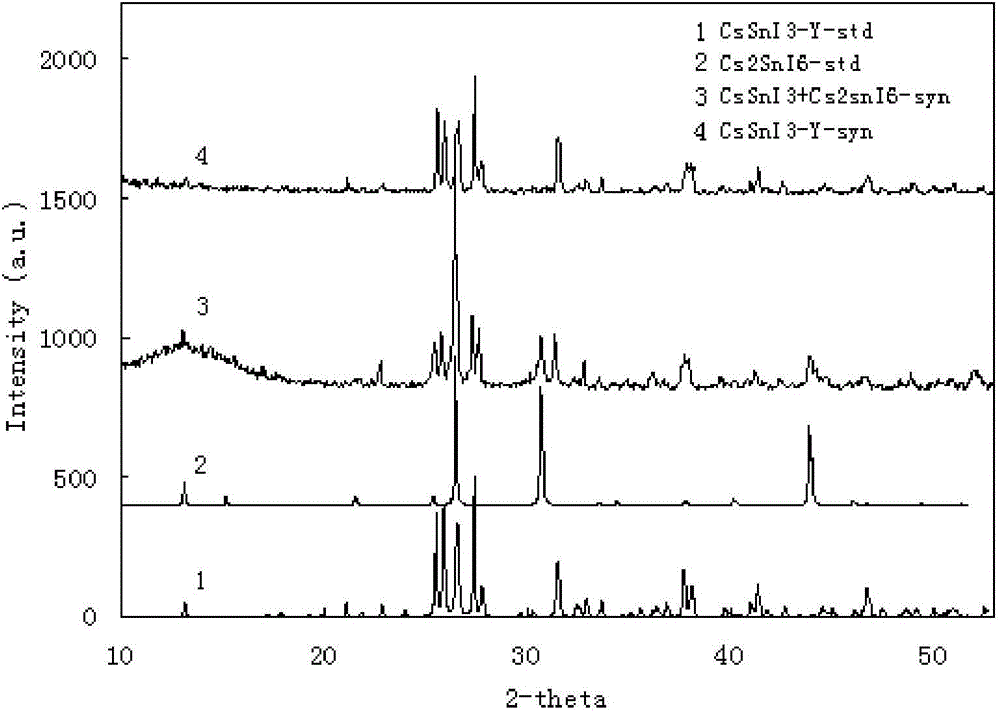
[0056] First, commercialized SnI2 (0.01 mol, 3.725 g) and CsI (0.01 mol, 2.598 g) were mixed in equimolar amounts, placed in a heating tube with a switch, and vacuumed for 40 minutes to remove the moisture in the tube React with air at 500°C for 45 minutes, then control the temperature and slowly cool down to room temperature within 5 hours to obtain a black solid. The material was used directly in the next preparation without treatment. Yield 100%. According to XRD analysis, the substance is composed of CsSnI3 ...
Embodiment 2
[0074] Preparation of High Purity CsSnBr3
[0075] 1. Synthesis of CsSnBr3 (Cs2SnBr6):
[0076] 1) Reaction formula:
[0077] SnBr2+CsBr---------CsSnBr3
[0078] SnBr4 (a small amount)+2CsB--------Cs2SnBr6 (a small amount)
[0079] 2) specific method
[0080] Similar to step 1(2) of Example 1. The difference is that the reactant SnBr2 replaces SnI2, and CsBr replaces CsI, wherein the amount of commercialized SnBr2 is 0.01 mole, 2.785 grams, and the amount of CsBr is 0.01 mole, 2.128 grams.
[0081] 2. Synthesis of CsSnBr3(Sn):
[0082] 1) Reaction formula:
[0083] CsSnBr3+Sn (excess) ------ no reaction
[0084] Cs2SnBr6 (a small amount) + Sn (excessive) ---- 2CsSnBr3 + Sn (excessive)
[0085] 2) Specific method:
[0086] The mixture (5.0 grams) of CsSnBr3 (Cs2SnBr6) obtained above was added with a small amount of Sn powder (0.1 grams), and placed in a heating tube together, and reacted at 450 degrees under vacuum for 30 minutes, and then slowly within 3 hours. After...
Embodiment 3
[0098] Preparation of High Purity CsSnCl3
[0099] 1. Synthesis of CsSnCl3 (Cs2SnCl6):
[0100] 1) Reaction formula:
[0101] SnCl2+CsCl---------CsSnCl3
[0102] SnCl4 (a small amount)+2CsCl-------Cs2SnCl6 (a small amount)
[0103] 2) specific method
[0104] First, commercialized SnCl2 (0.01 mol, 1.896 g) and CsCl (0.01 mol, 1.684 g) were mixed in equimolar amounts, placed in a heating tube with a switch, and vacuumed for 30 minutes to remove moisture in the tube With air, react at 550 degree temperature for 60 minutes, then control the temperature and slowly cool down to room temperature within 4 hours to obtain a black solid substance. The material was used in the next preparation without treatment. Yield 100%. According to XRD analysis, the substance is composed of CsSnCl3 and Cs2SnCl6.
[0105] 2. Synthesis of CsSnCl3(Sn):
[0106] 1) Reaction formula:
[0107] CsSnCl3+Sn (excess) ------ no reaction
[0108] Cs2SnCl6 (a small amount) + Sn (excessive) ---- 2CsSnC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com