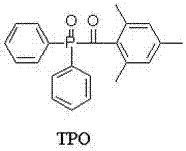
A kind of preparation method of 2,4,6 trimethylbenzoyl diphenyl phosphine oxide
A technology of trimethylbenzoyldiphenyl and triphenylphosphine, applied in the field of preparation of 2,4,6-trimethylbenzoyldiphenylphosphine oxide, which can solve the difficulty of recovery and high equipment requirements , Difficult to purify and other problems, to achieve the effect of easy industrialization, solving difficult and difficult preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 The preparation method of 2,4,6-trimethylbenzoyldiphenylphosphine oxide
[0033] 1) Under the protection of nitrogen, suspend metal sodium block (6.9g, 0.3mol) in 80ml toluene, heat until reflux, and stir vigorously to form a suspension of powdery sodium, then add triphenylphosphine dropwise under vigorous stirring (26.2g, 0.1mol), after dropping, heat to reflux for reaction, when the reaction of triphenylphosphine is complete, cool down to room temperature, add phosphorus trichloride (6.9g, 0.05mol) dropwise, stir vigorously during the dropwise addition After dropping, heat to 100°C to keep warm for reaction, and TLC to monitor the reaction;
[0034] 2) Cool the mixture obtained in step 1) to 80°C, add 2,4,6-trimethylbenzoyl chloride (27.4g, 0.15mol) dropwise at this temperature, and react after dropping, keep warm for the reaction, after the reaction is complete , add water to the reaction system, stir and react for 0.5h;
[0035] 3) Add 30% hydrogen perox...
Embodiment 2
[0036] Example 2 The preparation method of 2,4,6-trimethylbenzoyldiphenylphosphine oxide
[0037]1) Under the protection of nitrogen, suspend metal sodium block (6.9g, 0.3mol) in 80ml ethylbenzene, heat until reflux, stir vigorously, after forming a suspension of powdery sodium, add triphenyl triphenyl under vigorous stirring Phosphine (26.2g, 0.1mol), after dropping, heat to reflux for reaction, when the reaction of triphenylphosphine is complete, cool down to room temperature, add phosphorus trichloride (6.9g, 0.05mol) dropwise, stir vigorously during the dropwise addition Reaction, heating to 100°C for reaction after dropping, TLC monitoring reaction;
[0038] 2) Cool the mixture obtained in step 1) to 80°C, add 2,4,6-trimethylbenzoyl chloride (27.4g, 0.15mol) dropwise at this temperature, and react after dropping, keep warm for the reaction, after the reaction is complete , add water to the reaction system, stir and react for 0.5h;
Embodiment 3
[0040] Example 3 Preparation method of 2,4,6-trimethylbenzoyldiphenylphosphine oxide
[0041] 1) Under the protection of nitrogen, suspend metal sodium block (6.9g, 0.3mol) in 80ml toluene, heat until reflux, and stir vigorously to form a suspension of powdery sodium, then add triphenylphosphine dropwise under vigorous stirring (26.2g, 0.1mol), after dropping, heat to reflux for reaction, when the reaction of triphenylphosphine is complete, cool down to room temperature, add phosphorus trichloride (6.9g, 0.05mol) dropwise, stir vigorously during the dropwise addition After dropping, heat to 100°C to keep warm for reaction, and TLC to monitor the reaction;
[0042] 2) Cool the mixture obtained in step 1) to 80°C, add 2,4,6-trimethylbenzoyl chloride (31.1g, 0.17mol) dropwise at this temperature, and react after dropping, keep warm for the reaction, after the reaction is complete , add water to the reaction system, stir and react for 0.5h;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com