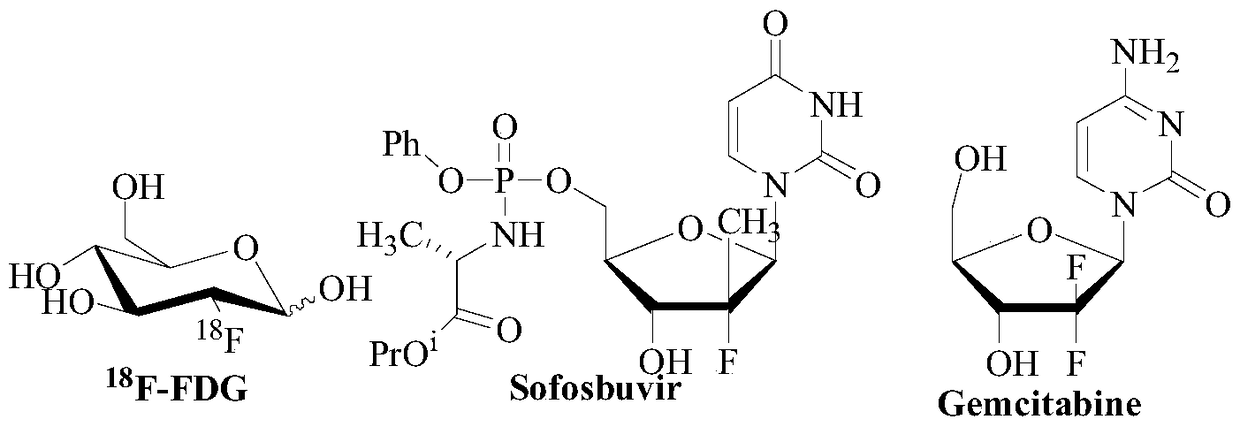
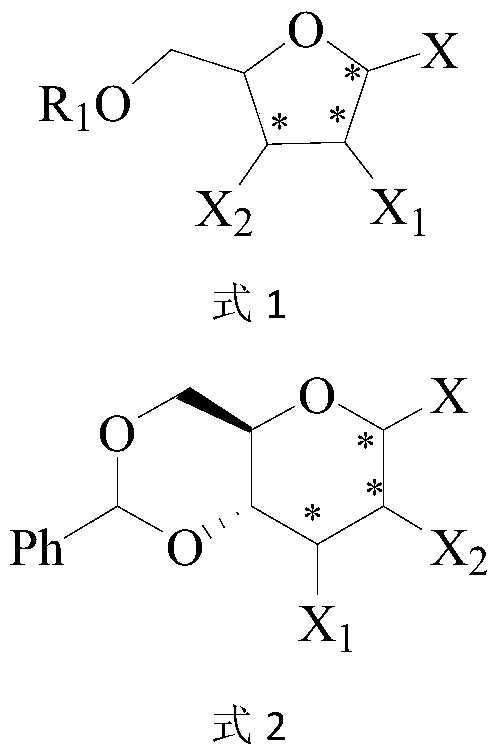
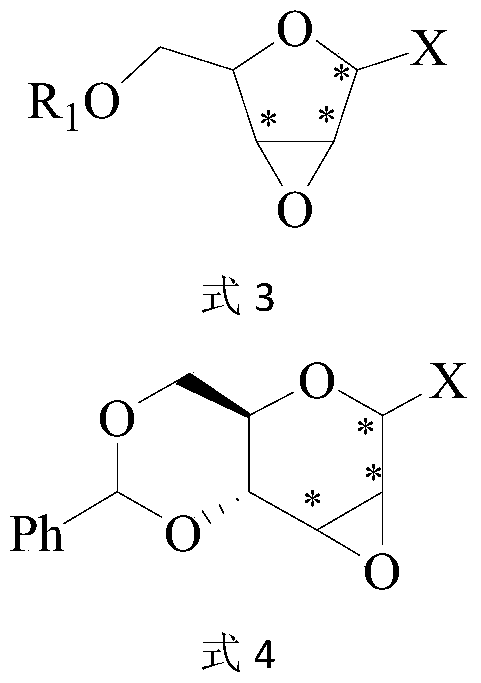
A kind of fluoroglycoside derivative and its preparation method
A technology of fluorinated glycosides and derivatives, which is applied in the field of synthesis of carbohydrate heterocyclic compounds, can solve the problems of harsh reaction conditions, expensive fluorinated reagents, and low fluorinated efficiency, and achieves simple methods, simple post-treatment process, and fluorinated high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] First refer to the literature [Veleti, S.K.; et al.J.Org.Chem, 2014, 79, 9444-9450; Wang, Yuan et al.Org.Lett, 2005, 7, 5577-5579; Kulkarni, S.S.; et al. J.Org.Chem, 2005,70,2808-2811.] Use whole acetylglucose as raw material to synthesize inner ether sugar (1a) through five-step reaction, and then TBAF·3H 2 O (1.26g, 4mmol, 2eq), KHF 2 (156mg, 2mmol, 1eq), the shrinkage endosaccharide compound 1a (712mg, 2mmol, 1equiv) was added to the reactor under the condition of isolated air; then the reactor was put into an oil bath and reacted at 70°C for 2h; after the reaction , the reaction solution was cooled to room temperature, diluted with ethyl acetate (50 mL), and washed with saturated sodium bicarbonate (10 mL) and water (5 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure and separated by silica gel flash column chromatography to obtain a single fluorinated product 1 (624 mg, 1.66...
Embodiment 2
[0048]
[0049] References [Yun, H.C.; Synthetic Communications, 2003, 33, 2349-2363; Anker, D.; Giudicelli, M.B.; Journal of Carbohydrate Chemistry, 1991, 10, 1991.]. As a raw material, the inner ether sugar (2a) is synthesized through a five-step reaction, and then TBAF·3H 2 O (1.26g, 4mmol, 2eq), KHF 2 (156mg, 2mmol, 1eq), and the shrinkage endosaccharide compound 2a (472mg, 2mmol, 1equiv) were added to the reaction kettle under the condition of isolated air; then the reaction kettle was put into an oil bath and reacted at 110°C for 3h; after the reaction , the reaction solution was cooled to room temperature, diluted with ethyl acetate (80 mL), and washed with saturated sodium bicarbonate (20 mL) and water (10 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure and separated by silica gel flash column chromatography to obtain a single fluorinated product 2 (417mg, 1.63mmol).
[0050]
[005...
Embodiment 3
[0053]
[0054] References [Coppola, Y.; Lorenzo, D.N.; J. Org. Chem. 2007, 72, 9679-9689; Wang, H.; She, J.; Zhang, L.-H.; Ye, X.-S.J. Org.Chem.2004,69,5774-5777.]. Using methyl-α-D-glucoside as raw material, the inner ether sugar (3a) was synthesized through four-step reaction, and then TBAF·3H 2 O (1.58g, 2mmol, 2eq), KHF 2 (156mg, 2mmol, 1eq), the shrinkage endosaccharide compound 3a (528mg, 2mmol, 1equiv) was added to the reaction kettle under the condition of isolated air; then the reaction kettle was put into an oil bath and reacted at 130°C for 2h; after the reaction , the reaction solution was cooled to room temperature, diluted with ethyl acetate (80 mL), and washed with saturated sodium bicarbonate (20 mL) and water (10 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure and separated by silica gel flash column chromatography to obtain a single fluorinated product 3 (477mg, 1.68mmol).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com