An Enhanced Composite Natural Gas Hydrate Inhibitor
A hydrate inhibitor and natural gas technology, applied in the direction of drilling compositions, chemical instruments and methods, etc., can solve the problems of low concentration, limited dispersion performance of polymerization inhibitors, and limited application occasions, so as to improve inhibition, Effects of reducing suppression costs and reducing environmental damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
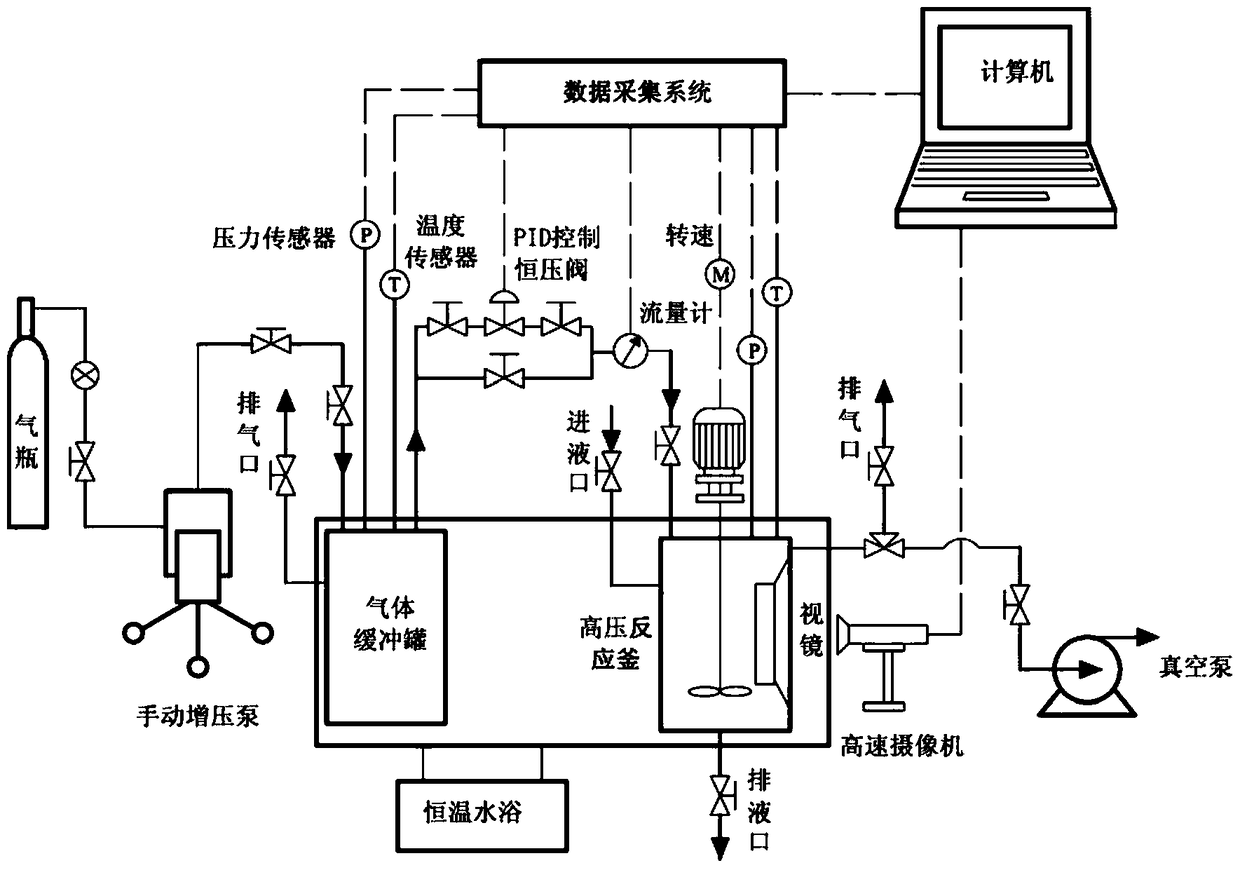
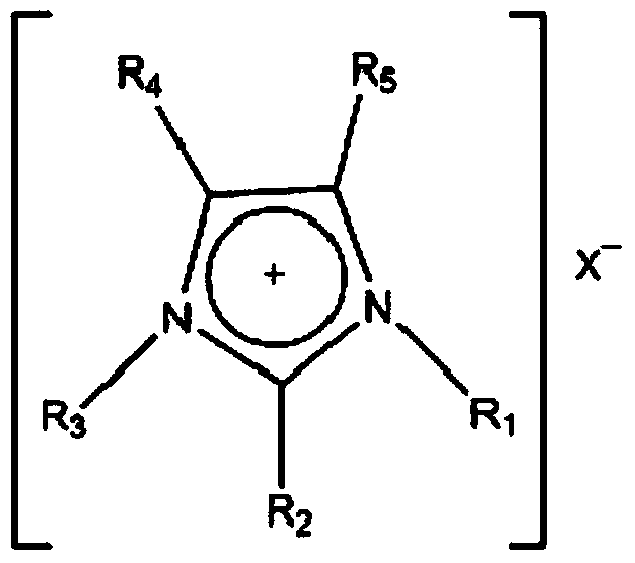
[0031] 1-Ethyl-3-methylimidazolium tetrafluoroborate ([Emim][BF 4 ]) and ethanol (EG) according to the mass ratio of 1:1 to prepare a mass concentration of 1% complex hydrate inhibitor aqueous solution. Put it into the reaction kettle, the experimental pressure is 10MPa, the experimental temperature is 276.15K, and the above experimental steps are followed.
[0032] The results show that the temperature rises and the pressure drops in the system at 4.9 hours, indicating that hydrates were formed at the above time (the formation of hydrates is an exothermic and gas-consuming process), but the stirring did not stop within 24 hours of the experiment . It shows that the 1% compound hydrate inhibitor has obvious hydrate inhibition effect and can withstand the supercooling degree above 15°C.
Embodiment 2
[0034] 1-Ethyl-3-methylimidazolium tetrafluoroborate ([Emim][BF 4 ]) and ethanol (EG) according to the mass ratio of 1:10 to prepare a mass concentration of 5.5% complex hydrate inhibitor aqueous solution. Put it into the reaction kettle, the experimental pressure is 10MPa, the experimental temperature is 276.15K, and the above experimental steps are followed.
[0035] The results show that the temperature rises and the pressure drops in the system at 9.5 hours, indicating that hydrates were formed at the above time (the formation of hydrates is an exothermic and gas-consuming process), but the stirring did not stop within 24 hours of the experiment . It shows that the 5.5% compound hydrate inhibitor has obvious hydrate inhibition effect and can withstand the supercooling degree above 15°C.
Embodiment 3
[0037] 1-Ethyl-3-methylimidazolium tetrafluoroborate ([Emim][BF 4 ]) and ethylene glycol ether (EGEE) according to the mass ratio of 1:10 to prepare a mass concentration of 5.5% complex hydrate inhibitor aqueous solution. Put it into the reaction kettle, the experimental pressure is 10MPa, the experimental temperature is 276.15K, and the above experimental steps are followed.
[0038] The results show that the temperature rises and the pressure drops in the system at 11.7 hours, indicating that hydrates were formed at the above time (the formation of hydrates is an exothermic and gas-consuming process), but the stirring did not stop within 24 hours of the experiment . It shows that the 5.5% compound hydrate inhibitor has obvious hydrate inhibition effect and can withstand the supercooling degree above 15°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com