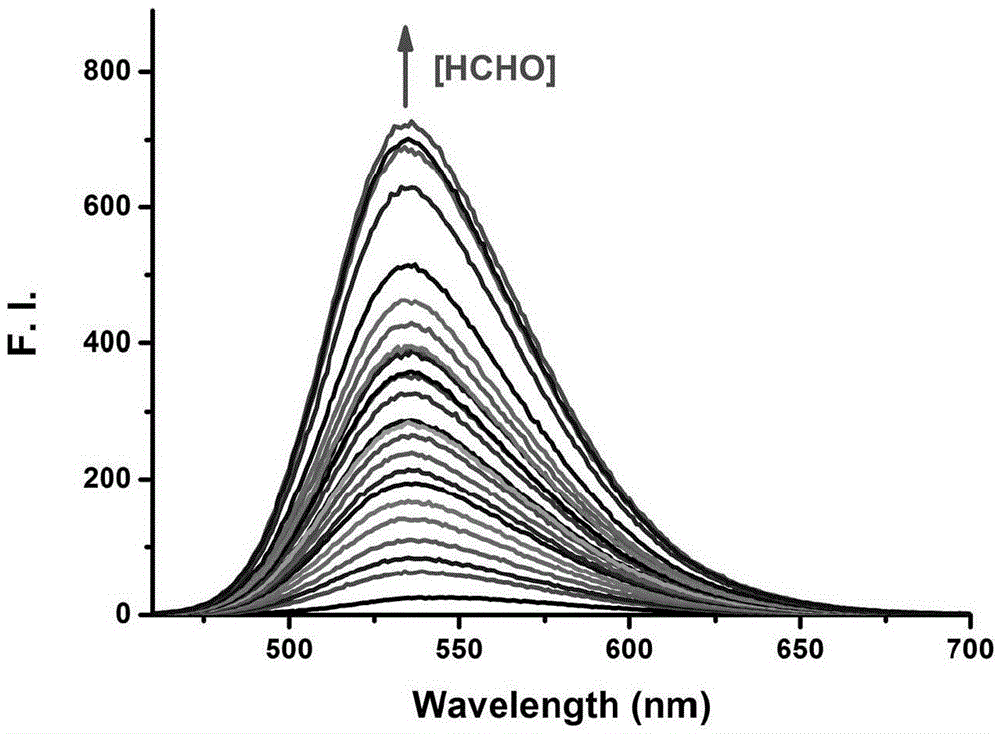
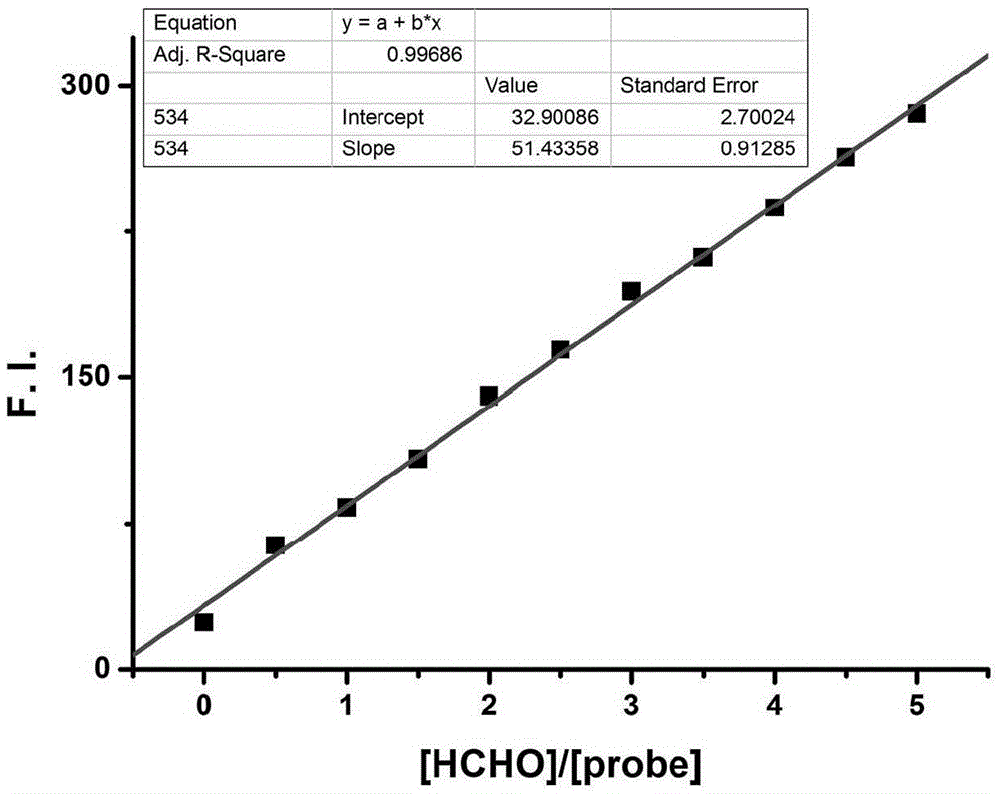
Fluorescent probe for detecting formaldehyde as well as preparation method and application of fluorescent probe
A fluorescent probe, formaldehyde technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of complex molecular structure of formaldehyde probe, slow reaction speed, lengthy synthesis steps, etc., and achieve easy popularization and application. , Easy to operate, the effect of improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
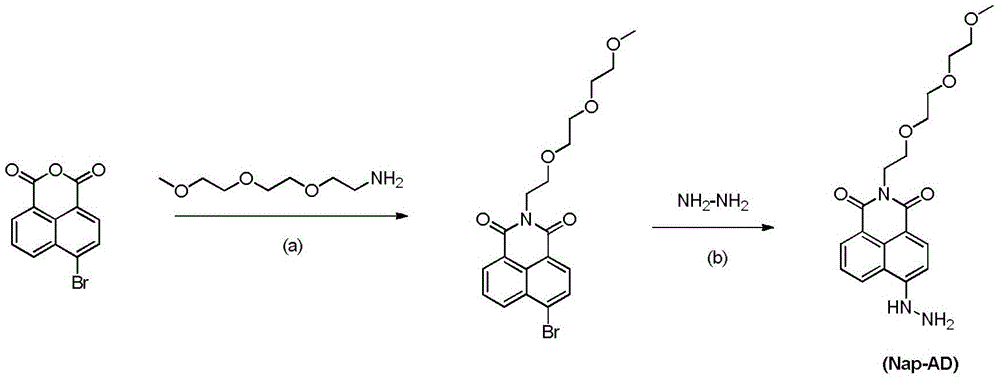
[0051]Embodiment 1, the preparation of fluorescent probe Nap-AD
[0052] Step a): under an inert atmosphere, add 5.00g of 4-bromo-1,8-naphthalic anhydride to 100mL of absolute ethanol, and then inject 2.95g of 3,6,9-trioxa-1-aminodecane, Reflux for 5 hours. After the reaction is complete, leave it overnight to precipitate needle-like crystals, filter and wash with cold ethanol three times to obtain the intermediate N-3,6,9-trioxadecanyl-4-bromo-1,8-naphthalimide 6.10 g (80% yield).
[0053] Step b): Under an inert atmosphere, add 5.00g of N-3,6,9-trioxadecanyl-4-bromo-1,8-naphthalimide and 3.79g of hydrazine hydrate into 100mL of acetone and reflux React for 12 hours. After the reaction was complete, the reaction solution was spin-dried, separated and purified by column chromatography to obtain 4.10 g (93% yield) of the final product Nap-AD, a yellow solid.
[0054] 1 HNMR (400MHz, Chloroform-d) δ8.07 (t, J = 8.3Hz, 2H), 7.74 (d, J = 8.3Hz, 1H), 7.29–7.11 (m, 2H), 6.93 (d...
Embodiment 2
[0055] Embodiment 2, the preparation of fluorescent probe Nap-AD
[0056] Step a): Under an inert atmosphere, add 5.00g of 4-bromo-1,8-naphthalene dicarboxylic anhydride into 100mL of anhydrous methanol, and then inject 1.96g of 3,6,9-trioxa-1-aminodecane, Reflux for 10 hours. After the reaction is complete, leave it overnight to precipitate needle-like crystals, filter and wash with cold ethanol three times to obtain the intermediate N-3,6,9-trioxadecanyl-4-bromo-1,8-naphthalimide 4.90 g (96% yield).
[0057] Step b): Under an inert atmosphere, add 5.00g of N-3,6,9-trioxadecanyl-4-bromo-1,8-naphthalimide and 1.52g of hydrazine hydrate into 100mL of acetonitrile and reflux React for 20 hours. After the reaction was complete, the reaction solution was spin-dried, separated and purified by column chromatography to obtain 4.10 g (93% yield) of the final product Nap-AD, a yellow solid.
[0058] 1 HNMR (400MHz, Chloroform-d) δ8.07 (t, J = 8.3Hz, 2H), 7.74 (d, J = 8.3Hz, 1H), 7...
Embodiment 3
[0059] Embodiment 3, the preparation of fluorescent probe Nap-AD
[0060] Step a): Under an inert atmosphere, add 5.00g of 4-bromo-1,8-naphthalic anhydride to 100mL of anhydrous ethylene glycol monomethyl ether, and then inject 1.47g of 3,6,9-trioxa-1 -Aminodecane, reflux reaction for 6 hours. After the reaction is complete, leave it overnight to precipitate needle-like crystals, filter and wash with cold ethanol three times to obtain the intermediate N-3,6,9-trioxadecanyl-4-bromo-1,8-naphthalimide 3.60 g (95% yield).
[0061] Step b): Under an inert atmosphere, add 5.00g of N-3,6,9-trioxadecanyl-4-bromo-1,8-naphthalimide and 0.76g of hydrazine hydrate into 100mL DMF for reflux reaction 1 hour. After the reaction was complete, the reaction solution was spin-dried, separated and purified by column chromatography to obtain 4.30 g (97% yield) of the final product Nap-AD as a yellow solid.
[0062] 1 HNMR (400MHz, Chloroform-d) δ8.07 (t, J = 8.3Hz, 2H), 7.74 (d, J = 8.3Hz, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com