Topical compositions for treatment of excessive sweating and methods of use thereof
一种组合物、化合物的技术,应用在药物组合、医药配方、梳妆用配制品等方向,能够解决未满足医疗需求等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0240] Embodiment 1: pharmaceutical composition
[0241] The following compositions were prepared as indicated in the table below.
[0242] Table 1:
[0243]
[0244]
[0245] Formulation Nos. 1, 3, 4, 6, and 8 (solutions) were prepared by adding each solvent shown in Table 1 to a mixing vessel while stirring. Uclidinium was then added to the mixing vessel while stirring to obtain the desired solution formulation.
[0246] Formulation Nos. 2, 5, 7, 9 and 10 (gels) were prepared by adding each solvent shown in Table 1 to a mixing vessel while stirring. The umeclidinium is then added to the mixing vessel while stirring, followed by the addition of the hydroxypropyl cellulose to obtain the desired gel formulation.
[0247]The following additional formulations were also prepared to measure the viscosity of gel formulations according to the invention with different levels of gelling agent (0, 1%, 1.5% and 2% Klucel-MF):
[0248] Table 2:
[0249]
[0250]
[0251] ...
Embodiment 2
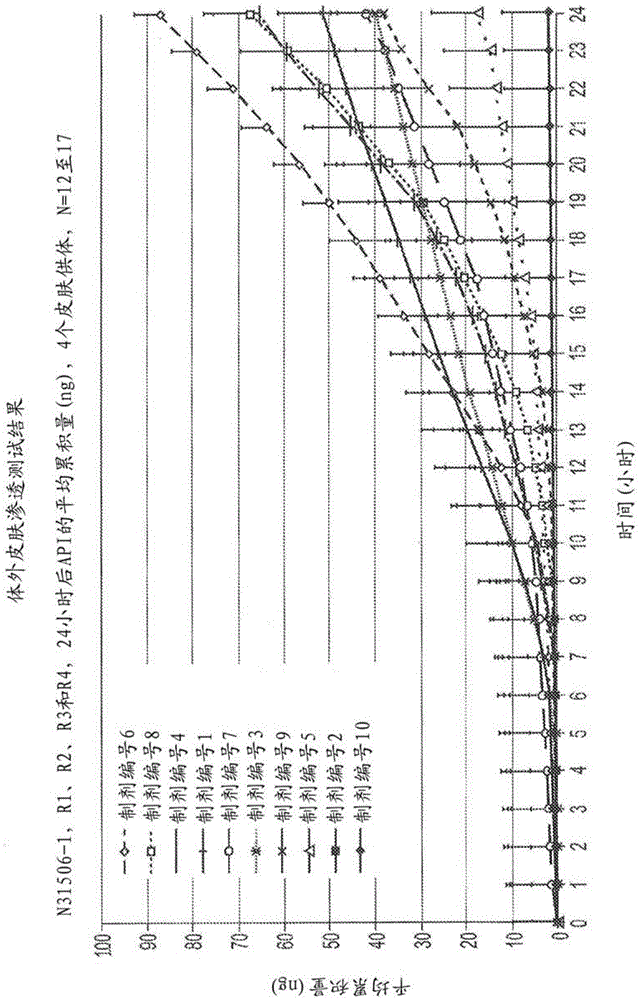
[0253] Example 2 - In Vitro Skin Penetration Study
[0254] The topical pharmaceutical composition described in Example 1 was subjected to an in vitro skin penetration study to measure skin flux. Use the following method:
[0255] Methods and Materials
[0256] Full thickness human skin was obtained from the patient via abdominoplasty at a local hospital. Immediately after collection the skin was transferred to a plastic container with phosphate buffered saline (PBS) and kept at 4°C for shipment. Once at the laboratory, subcutaneous fat is removed from the skin sample. Full-thickness skin was then placed on a block of high-density foam and harvested to a thickness of 500 μm using an Electro-Dermatome. The split thickness skin was then spread on aluminum foil and placed in a water impermeable plastic bag. The air is removed and the bag is heat sealed. Samples were stored at -80°C until the time of experiment. Previous experiments have shown that skin samples can be pre...
Embodiment 3
[0276] Example 3-in vitro skin penetration study (umeclidinium versus glycopyrronium bromide)
[0277] The in vitro study investigated the skin distribution (epidermal / dermal) and in vitro skin flux of the active ingredient delivered from (i) formulation number 1 shown in Table 1 containing 2.2% umeclidinium bromide and (ii) containing 2 % Glycopyrronium Bromide Comparative Formulation.
[0278] After administration of a single limited topical dose of umeclidinium bromide or glycopyrronium bromide on isolated human skin at 6 hours, the median value of the molar ratio of glycopyrronium bromide to umeclidinium bromide (after correcting for dose differences) was ( range) was 1.5 (0.4-5.8) in the epidermis and 1.2 (0.3-4.3) in the dermis. Thus, the amount of umeclidinium delivered to the dermis was of the same order (but on average slightly lower) than the amount of glycopyrronium bromide delivered to the dermis, based on normalized molar doses.
[0279] Following a single lim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com