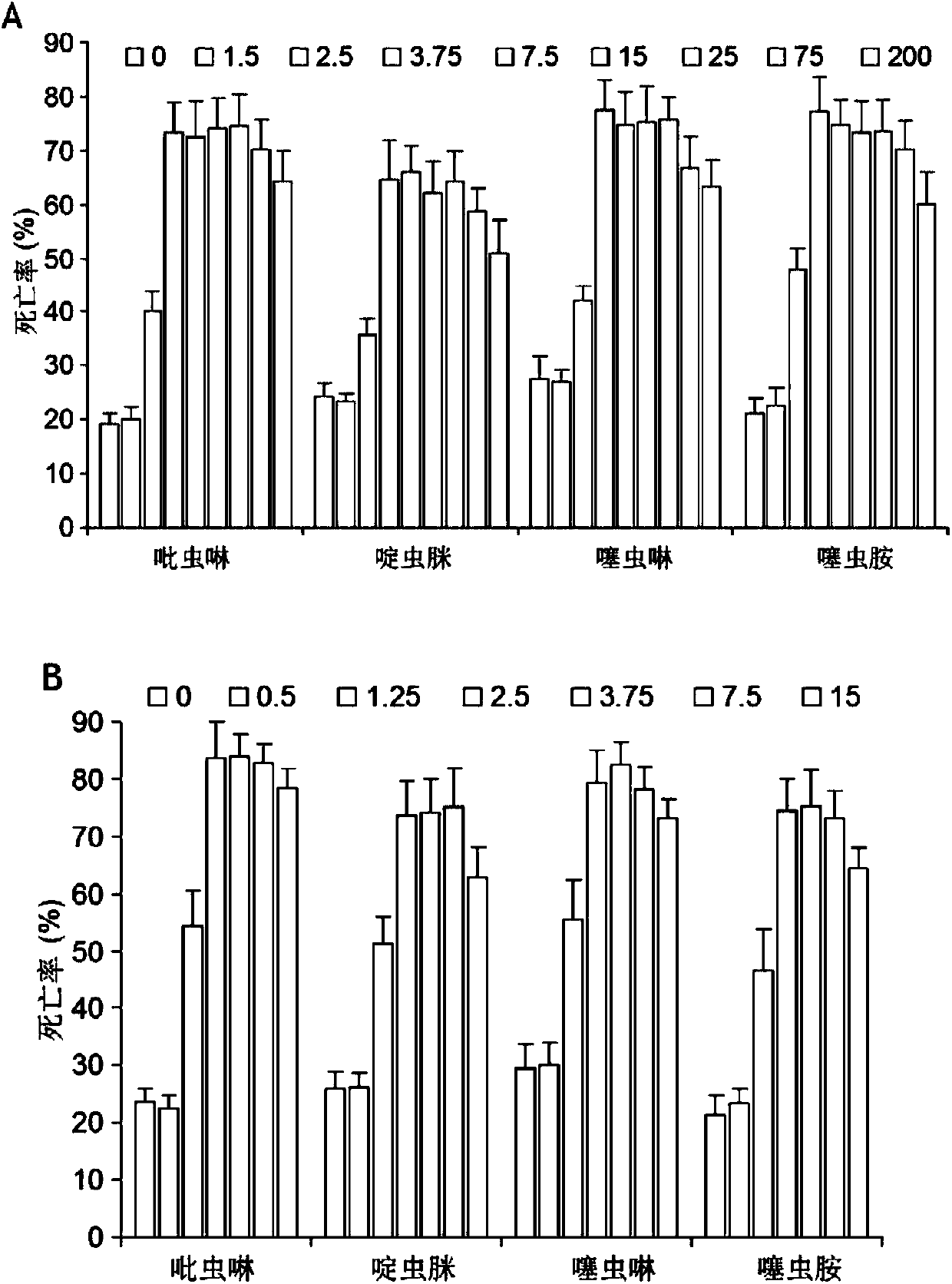
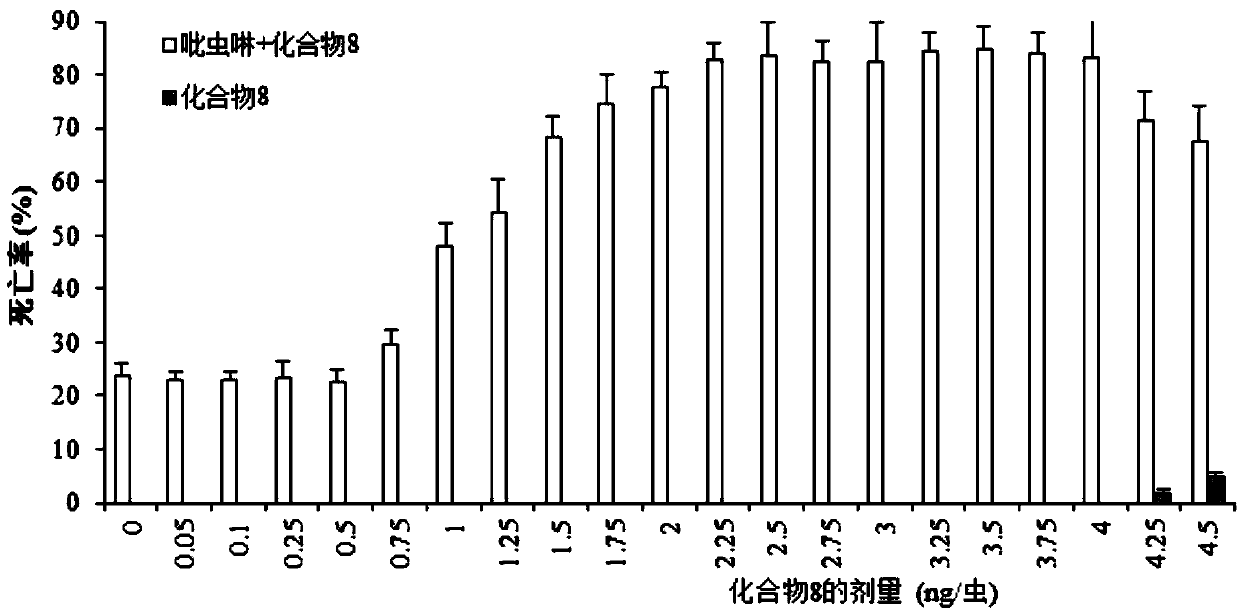
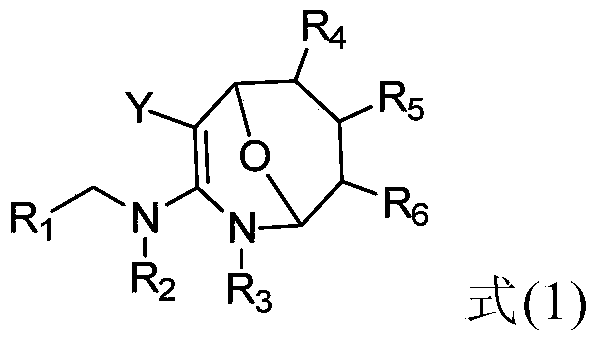
Application of eight-membered oxygen-bridge heterocyclic compound as pesticide synergist
A kind of technology of heterocyclic compound and eight-membered oxygen bridge, applied in the field of pesticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1: 1-(4-chlorophenyl)-10-nitro-1,2,3,5,6,7,8,9-octahydro-5,9-epoxyimidazo[1,2 -a] Synthesis of azacyclooctene tetraene (compound 9)
[0091]
[0092] Put 1.27g (0.005mol) of 1-(4-chlorophenyl)-2-(nitromethylene) imidazolidine, 30ml of anhydrous acetonitrile, 3ml of 25% glutaraldehyde aqueous solution, and a catalytic amount of HCl in 50ml in a round bottom flask. Stir at room temperature, and track the reaction by TLC. After the reaction was finished, the solvent was removed, and the pure product was obtained as light yellow powder through column chromatography separation, with a yield of 86%. mp=174.7-175.4°C; 1 HNMR (400Mz, DMSO-d 6):δ7.39(s,306H),7.32(s,307H),4.84(s,308H),4.73(s,154H),4.46(s,158H),3.22(s,80H),3.14(s, 86H), 2.89(s, 87H), 2.84(s, 82H), 1.94(t, J=3.5Hz, 30H), 1.93(s, 148H), 1.77(dd, J=65.0, 60.0Hz, 590H), 1.56(d, J=80.0Hz, 257H), 1.46(d, J=1.6Hz, 6H)ppm; 13 CNMR (100Mz, DMSO-d 6 ): δ167.96, 136.07, 132.68, 129.50, 128.21, 80.05, 64.91...
Embodiment 2
[0093] Example 2: 1-(3-fluoropropyl)-1,2,3,5,6,7,8,9-octahydro-5,9-epoxyimidazolium [1,2-a] nitrogen heterocycle Synthesis of octene-10-carbonitrile (compound 10):
[0094]
[0095] Add 0.845 g (5.0 mmol) of 2-(1-(3-fluoropropyl)imidazoline-2-ylide) acetonitrile and 3 ml of 25% glutaraldehyde aqueous solution into 20 ml of acetonitrile, add a catalytic amount of concentrated hydrochloric acid, stir at room temperature, TLC traced, after the reaction was finished, the solvent was removed, and the pure product was obtained as a yellow powder through column chromatography separation, with a yield of 64%. 1 HNMR (400MHz, DMSO-d 6 )δ4.94(s,8H),5.95–2.81(m,74H),3.23(s,4H),3.10(s,8H),3.41–2.81(m,36H),2.96(d,J=17.4Hz ,14H),2.80(s,4H),1.93(s,6H),1.83(s,9H),1.73(d,J=4.0Hz,14H),1.71–1.46(m,24H)ppm; 13 CNMR (100MHz, DMSO-d 6 ): δ151.48, 120.64, 80.76, 80.05, 65.24, 61.05, 52.63, 49.19, 47.77, 31.54, 30.45, 26.78, 15.74ppm; HRMS(ES+)C 13 h 19 FN 3 O(M+H) + , calculated value: 25...
Embodiment 3
[0096] Example 3: 1-Benzyl-10-nitro-1,2,3,5,6,7,8,9-octahydro-5,9-epoxyimidazo[1,2-a]azepine Synthesis of Cyclooctene (Compound 6)
[0097]
[0098] Put 1.10 g (0.005 mol) of 1-benzyl-2-(nitromethylene) imidazolidine, 30 ml of anhydrous acetonitrile, 3 ml of 25% glutaraldehyde aqueous solution, and a catalytic amount of HCl in a 50 ml round bottom flask . Stir at room temperature, and track the reaction by TLC. After the reaction was finished, the solvent was removed, and the pure product was obtained as light yellow powder through column chromatography separation, with a yield of 66%. mp=154.7-155.4°C; 1 HNMR (400Mz, DMSO-d 6 ):δ7.36(s,82H),7.29(d,J=15.0Hz,170H),4.84(s,110H),4.74(s,55H),4.60(s,58H),3.18(s,30H) ,3.08(s,31H),2.80(d,J=13.8Hz,85H),1.94(t,J=3.5Hz,11H),1.93(s,53H),1.77(dd,J=65.0,60.0Hz, 211H), 1.56 (d, J=80.0Hz, 92H) ppm; 13 CNMR (100Mz, DMSO-d 6 ): δ167.96, 137.34, 128.64, 128.56, 127.89, 80.05, 64.91, 54.33, 50.62, 47.77, 31.93, 31.54, 15.74ppm; HRMS (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com