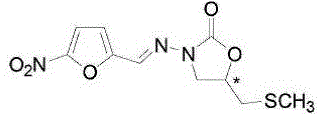
Nifuratel solid preparation and preparation method thereof
A technology of nifuratel solid and nifuratel, which is applied in the field of nifuratel solid dispersion and nifuratel tablets and its preparation, can solve the problem of low dissolution rate and low bioavailability of nifuratel preparations and other issues, to achieve the effect of improving bioavailability, high bioavailability, and ensuring drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
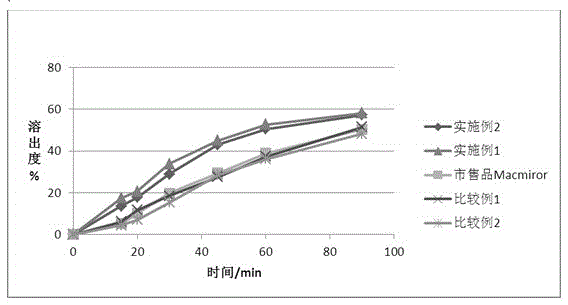
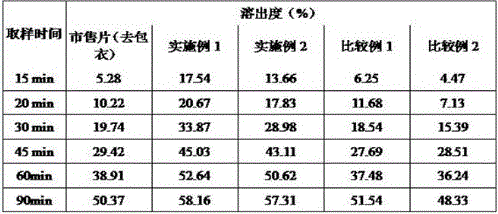
Image
Examples
Embodiment 1
[0022] (1) Preparation of solid dispersion: Weigh the nifuratel raw material drug and mannitol in the prescription amount shown in Table 1, and use a ball mill to perform ball milling and mixing for 45 minutes to obtain a nifuratel solid dispersion.
[0023] (2) Preparation of soft material: mix the solid dispersion obtained in step (1) with the starch in the prescription amount shown in Table 1, and add 35-40g of water to prepare soft material.
[0024] (3) Granulation and drying: pass the soft material obtained in step (2) through a 24-mesh sieve to granulate, and then dry in an oven at 60°C for 1 hour.
[0025] (4) Grain sizing: pass the dry granules obtained in step (3) through a 20-mesh sieve for sizing.
[0026] (5) Mixing and tableting: Mix the granules obtained in step (4) with the prescription amounts shown in Table 1, talcum powder and magnesium stearate evenly, and press into tablets.
[0027] (6) Coating: prepare a coating liquid, coat plain tablets, and get ready...
Embodiment 2
[0031] (1) Preparation of solid dispersion: Weigh the nifuratel raw material drug and PEG-6000 in the prescription amount shown in Table 2, and use a ball mill to perform ball milling and mixing for 60 minutes to obtain a nifuratel solid dispersion.
[0032] (2) Preparation of soft material: mix the solid dispersion obtained in step (1) with the starch in the prescription amount shown in Table 2, and add 30-35g of water to prepare soft material.
[0033] (3) Granulation and drying: Pass the soft material obtained in step (2) through a 20-mesh sieve to granulate, and then dry it in an oven at 60°C for 1 hour.
[0034] (4) Grain sizing: pass the dry granules obtained in step (3) through a 20-mesh sieve for sizing.
[0035] (5) Mixing and tableting: Mix the granules obtained in step (4) with talcum powder and magnesium stearate in the prescription amounts shown in Table 2, and then press them into tablets.
[0036] (6) Coating: prepare a coating liquid, coat plain tablets, and g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com