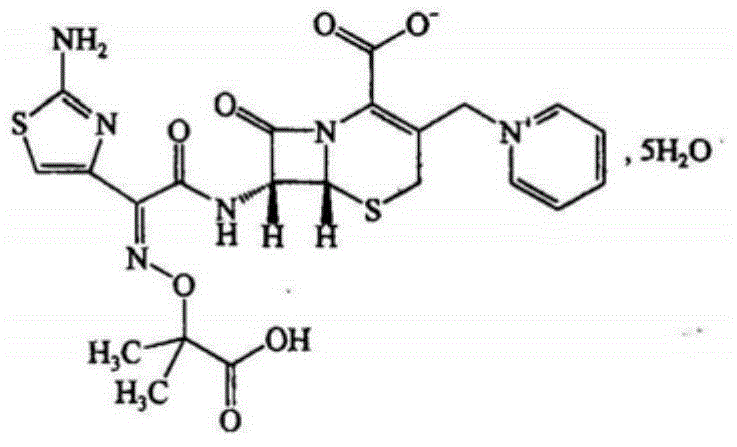
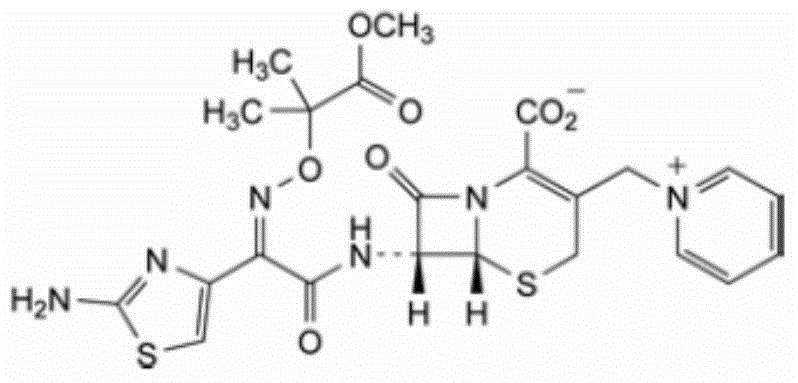
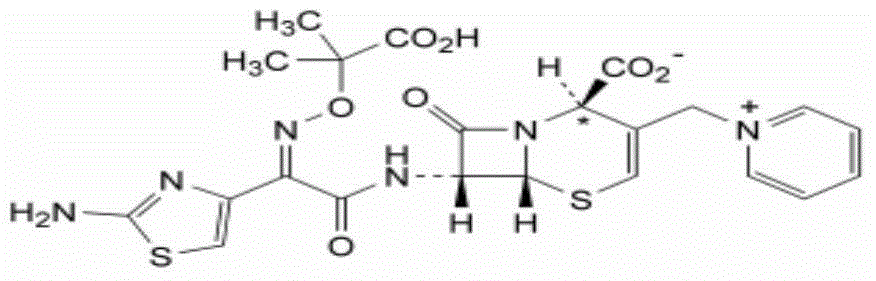
High-purity ceftazidime powder injection and preparation method thereof
A ceftazidime, high-purity technology, applied in the field of high-purity ceftazidime powder injection and preparation thereof, can solve the problems of not being able to obtain ceftazidime and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Activated carbon (Egret A type) (its specific surface 1020m 2 / g, pore volume 0.60ml / g, average pore diameter 2.35nm) 200 grams are suspended in 1000 milliliters of water, stir, filter in the cylindrical funnel that diameter is 5cm, rinse gac with 1500 milliliters of water, vacuum-dry, form diameter is Cylindrical filter with 5cm activated carbon filter layer.
[0057] 50 g of ceftazidime circulating in the market was added to 100 ml of water, and 2N sodium hydroxide solution was added dropwise to dissolve it to obtain an aqueous solution of ceftazime, the pH of which was 6.3 to 7.0. Slowly add the above ceftazidime solution to the filter of the activated carbon filter layer, and add it in about 15 minutes. It was then eluted with 500 ml of water. Accept the filtrate, discard the first 100ml of filtrate, start collecting when the concentration of the filtrate reaches 30mg / L, control the flow rate at 4-5mL / min, stop collecting when the concentration of the filtrate is ...
Embodiment 2
[0067] Activated carbon (ENO activated carbon (its specific surface 1430m 2 / g, pore volume 1.17ml / g, average pore diameter 3.27nm) mixed with Egret A activated carbon 1:1) 300 grams suspended in 1000 milliliters of water, stirred, filtered into a cylindrical funnel with a diameter of 5 cm, with 2000 milliliters of water Rinse the activated carbon and vacuum dry to form a cylindrical filter with a diameter of 5 cm activated carbon filter layer.
[0068]50 grams of ceftazidime circulating in the market were added to 150 milliliters of water, and triethylamine was added dropwise to dissolve it to obtain an aqueous solution of ceftazidime, the pH of which was 5.5 to 6.0. Slowly add the above ceftazidime solution to the cylindrical filter of the activated carbon filter layer, and add it in about 20 minutes. It was then eluted with 500 ml of water. Accept the filtrate and discard the first 100 ml of filtrate. Then start to receive 450 ml of filtrate.
[0069] Adjust the tempera...
Embodiment 3
[0082] Preparation of ceftazidime powder injection
[0083] 9 kg of ceftazidime sterile powder bulk drug obtained by the method described in Example 1 and 1 kg of sodium carbonate sterile powder were mixed uniformly, and then packed to obtain sterile powder injection, specification 2.0 g / bottle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com