Pyridine-quinoline derivative, preparation method and organic light-emitting device
A technology of quinolines and derivatives, which is applied in the field of organic optoelectronic materials, can solve the problems of single species and difficult performance optimization, and achieve the effect of low manufacturing cost and reduced manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Add 100mmol of 4,9-dichloropyrido[2,3-g]quinoline and 220mmol of 4-(N,N-diphenyl)aminophenylboronic acid into a 1L three-necked flask, dissolve in 400mL of toluene, and Under the protection of nitrogen, add tetrakistriphenylphosphine palladium 1.5mmol, potassium carbonate 250mmol, distilled water 100mL, stir and reflux for 16 hours, cool to room temperature after the reaction, separate liquid, pass through a silica gel funnel, wash, spin dry, recrystallize, filter, 77 mmol of white solid compound 1 was obtained, yield 77%.
[0037] Embodiment 2: the synthesis of compound 2
Embodiment 2
[0038]
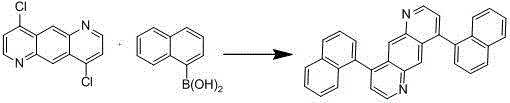
[0039] Add 100mmol of 4,9-dichloropyrido[2,3-g]quinoline and 220mmol of 1-naphthaleneboronic acid into a 1L three-necked flask, dissolve in 400mL of toluene, and add tetrakistriphenylphosphine palladium under nitrogen protection 1.5mmol, potassium carbonate 250mmol, distilled water 100mL, stirred and refluxed for 16 hours, cooled to room temperature after the reaction, separated, passed through a silica gel funnel, washed, spin-dried, recrystallized, filtered to obtain 87mmol of white solid compound 2, yield 87%.
[0040] Embodiment 3: the synthesis of compound 3
Embodiment 3
[0041]
[0042] Add 100mmol of 4,9-dichloropyrido[2,3-g]quinoline and 220mmol of 9-phenanthrene boronic acid into a 1L three-neck flask, dissolve in 400mL of toluene, and add tetrakistriphenylphosphine palladium under nitrogen protection 1.5mmol, potassium carbonate 250mmol, distilled water 100mL, stirred and refluxed for 16 hours, cooled to room temperature after the reaction, separated, passed through a silica gel funnel, washed, spin-dried, recrystallized, filtered to obtain 79mmol of white solid compound 3, yield 79%.
[0043] Embodiment 4: the synthesis of compound 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com