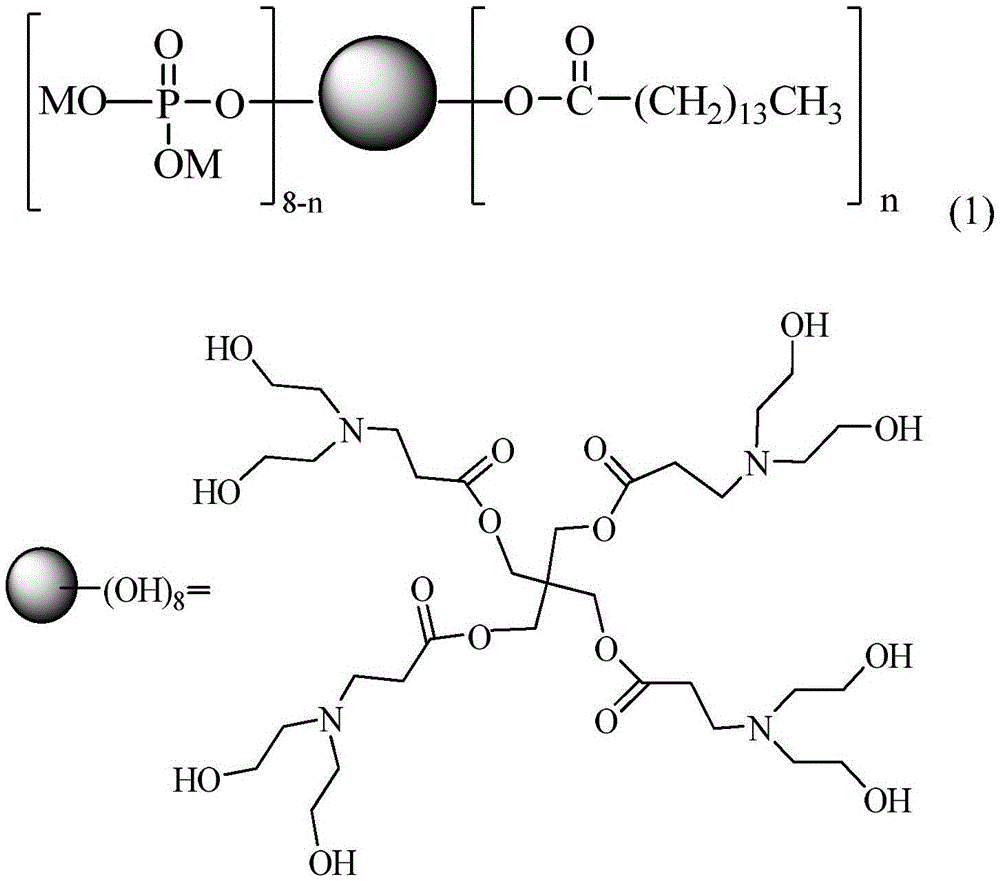
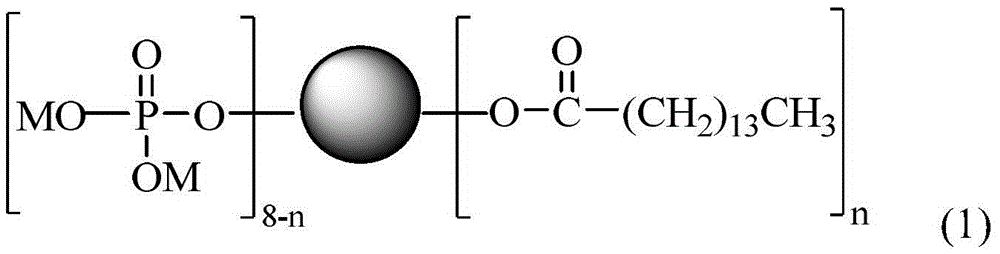Linear-hyperbranched phosphate salt surface active agent and preparation method thereof
A technology of surfactants and phosphate ester salts, applied in chemical instruments and methods, organic chemistry, phosphorus organic compounds, etc., can solve the problems of surfactant application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
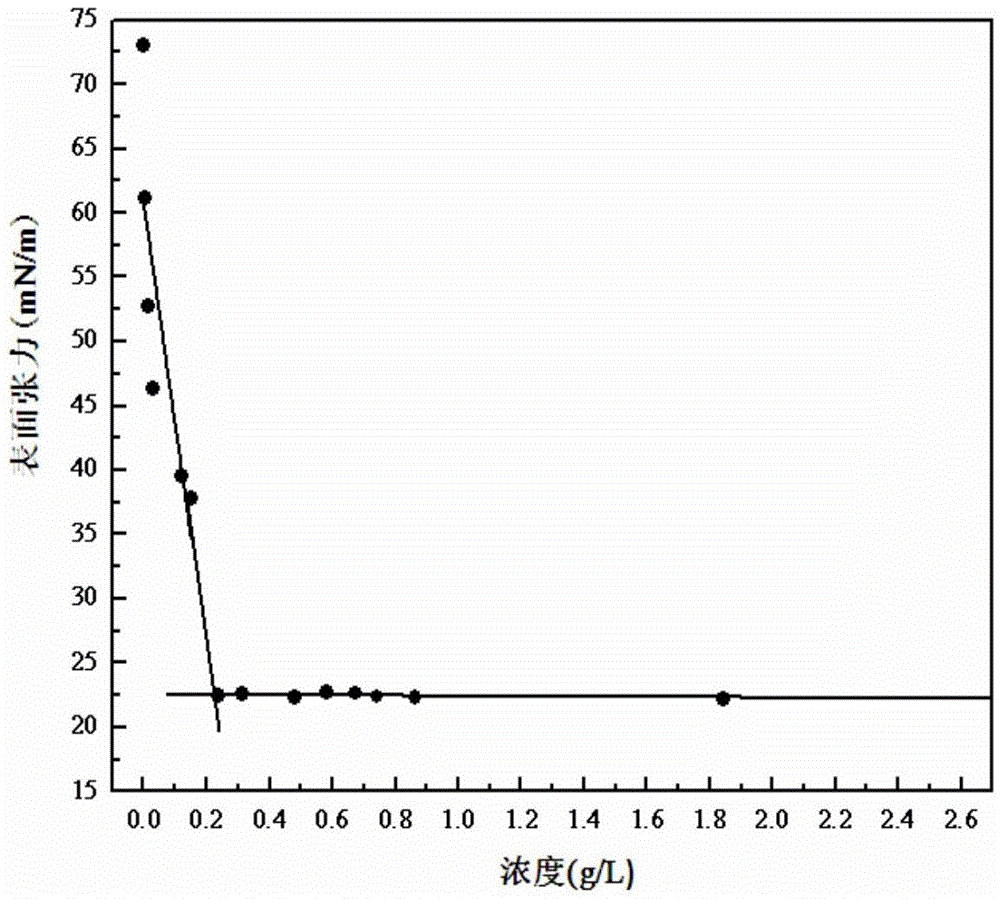
Image
Examples
Embodiment 1
[0037] 1. Preparation of hydroxyl-terminated hyperbranched polymers:
[0038] Add diethanolamine and methyl acrylate in a molar ratio of 1:2 to a reactor containing 10 mL of methanol solution, raise the temperature to 30°C, keep the temperature for 4 hours, remove methanol and excess methyl acrylate by vacuuming, and prepare AB 2 Monomer (methyl N,N-dihydroxyethyl-3-aminopropionate).
[0039] In the monomer prepared above, add pentaerythritol, the molar ratio of pentaerythritol to monomer is 1:4, then add p-toluenesulfonic acid with a total mass fraction of 0.5% in the reactor, mix and heat to 110°C, mix well After heating up to reflux, react for 6h, after cooling the reaction, dissolve in CH 2 Cl 2 Washing, suction filtration under reduced pressure for 3 times, and rotary evaporation to produce a hydroxyl-terminated hyperbranched polymer.
[0040] 2. Preparation of linear-hyperbranched polymers:
[0041] Add 50mL of pyridine solution in the reactor, remove the moisture in...
Embodiment 2
[0045] 1. Preparation of hydroxyl-terminated hyperbranched polymers:
[0046]Add diethanolamine and methyl acrylate in a molar ratio of 1:3 to a reactor containing 15 mL of methanol solution, raise the temperature to 50°C, keep the temperature for 5 hours, remove methanol and excess methyl acrylate by vacuuming, and prepare AB 2 Monomer (methyl N,N-dihydroxyethyl-3-aminopropionate).
[0047] Add pentaerythritol to the monomer prepared above, the molar ratio of pentaerythritol to monomer is 1:4, then add concentrated sulfuric acid with a total mass fraction of 1.0% in the reactor, mix and heat to 120°C, mix well and then heat up Reflux, react for 6h, after cooling the reaction, dissolve in CH 2 Cl 2 Washing, suction filtration twice under reduced pressure, and rotary evaporation to produce a hydroxyl-terminated hyperbranched polymer.
[0048] 2. Preparation of linear-hyperbranched polymers:
[0049] Add 60mL of pyridine solution in the reactor, remove the moisture in the re...
Embodiment 3
[0053] 1. Preparation of hydroxyl-terminated hyperbranched polymers:
[0054] Add diethanolamine and methyl acrylate in a molar ratio of 1:2 to a reactor containing 15 mL of methanol solution, raise the temperature to 35°C, keep the temperature for 4 hours, remove methanol and excess methyl acrylate by vacuuming, and prepare AB 2 Monomer (methyl N,N-dihydroxyethyl-3-aminopropionate).
[0055] In the monomer prepared above, add pentaerythritol, the molar ratio of pentaerythritol to monomer is 1:5, then add hydrochloric acid with a total mass fraction of 0.6% in the reactor, mix and heat to 120°C, mix well and then heat up to reflux , reacted for 6h, cooled the reaction, dissolved in CH 2 Cl 2 Washing, suction filtration under reduced pressure for 3 times, and rotary evaporation to produce a hydroxyl-terminated hyperbranched polymer.
[0056] 2. Preparation of linear-hyperbranched polymers:
[0057] Add 60mL of pyridine solution in the reactor, remove the moisture in the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com