Hydrolysis method of astilbin
A technology of astilbin and hydrolyzate, which is applied in the field of natural medicinal chemistry, can solve the problems of increased production costs, pollution, waste of resources and the environment, and achieve the effects of reducing waste of resources and environmental pollution, facilitating industrial production, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
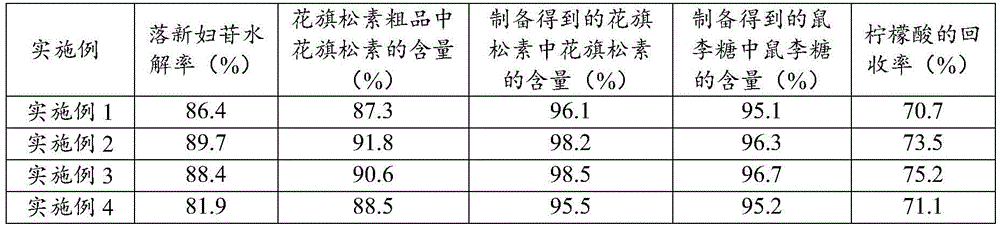
[0035] The hydrolysis method of astilbin in the present embodiment comprises the following steps of hydrolyzing astilbin to prepare taxifolin:
[0036] (1) Take 50 g of astilbin, add 350 mL of 2.5 mol / L citric acid aqueous solution under stirring, and hydrolyze at 90 °C for 3.5 h to obtain a hydrolyzed solution;
[0037] (2) filtering the hydrolyzate while hot, concentrating the filtrate to 200mL, standing for at least 12h, crystallizing, filtering, collecting the filter cake and the filtrate respectively, and the filter cake is the taxifolin crude product;
[0038] (3) The taxifolin crude product is recrystallized, and the specific conditions for recrystallization are as follows: the taxifolin crude product is dissolved in 40° C. water, and then cooled to room temperature to obtain taxifolin.
[0039] The hydrolysis method of astilbin in this embodiment also includes the following steps of hydrolyzing astilbin to prepare rhamnose: the above-mentioned filtrate is purified by A...
Embodiment 2
[0042] The hydrolysis method of astilbin in this embodiment includes the following steps of hydrolyzing astilbin to prepare taxifolin: (1) taking 50 g of astilbin, adding 500 mL of 1.5 mol / L citric acid aqueous solution under stirring, and hydrolyzing at 105°C for 3 hours, to obtain a hydrolyzate;
[0043] (2) the hydrolyzate was filtered while hot, the filtrate was concentrated to 190mL, left standing for at least 12h, crystallized, filtered, and the filter cake and the filtrate were collected respectively, and the filter cake was the taxifolin crude product;
[0044] (3) The taxifolin crude product is recrystallized, and the specific conditions of recrystallization are as follows: the taxifolin crude product is dissolved in 80° C. water, and then cooled to room temperature to obtain taxifolin.
[0045] The above filtrate is purified by AB-8 macroporous resin column chromatography, using water as the eluent, collecting the eluate, concentrating to Brix of 54-56%, and standing...
Embodiment 3
[0049] The hydrolysis method of astilbin in the present embodiment comprises the following steps of hydrolyzing astilbin to prepare taxifolin:
[0050] (1) Take 50 g of astilbin, add 750 mL of 1.0 mol / L citric acid aqueous solution under stirring, and hydrolyze at 115 °C for 2 h to obtain a hydrolyzed solution;
[0051] (2) filtering the hydrolyzate while hot, concentrating the filtrate to 210mL, standing for at least 12h, crystallizing, filtering, collecting the filter cake and the filtrate respectively, and the filter cake is the taxifolin crude product;
[0052] (3) The taxifolin crude product is recrystallized, and the specific conditions for recrystallization are as follows: the taxifolin crude product is dissolved in 60° C. water, and then cooled to room temperature to obtain taxifolin.
[0053] The hydrolysis method of astilbin in this embodiment also includes the following steps of hydrolyzing astilbin to prepare rhamnose: the above-mentioned filtrate is purified by AB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com