Esterase and application thereof
An esterase and reaction technology, applied in the field of industrial microorganisms, can solve problems such as cloning and expression to genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] This example is the cloning of the esterase gene of the present invention and the construction of Escherichia coli engineering bacteria.
[0022] 1. Extraction of BacillusamyloliquefaciensATCC23350DNA
[0023] The Bacillus amyloliquefaciens ATCC23350 bacterial strain was cultivated in LB medium for 12 hours, centrifuged at 12000r / min for 10 minutes to obtain the thallus, and the bacterial genome DNA extraction kit (TaKaRa company) was used to extract the Bacillus amyloliquefaciens ATCC23350 bacterial genome total DNA according to its operation, and put it in the refrigerator for later use.
[0024] 2. Competent preparation of Escherichia coli
[0025] (1) Inoculate E. coli DH5α and BL21(DE3) into 250 mL shake flasks containing 20 mL of LB medium, respectively, and culture overnight at 37° C. and 200 rpm / min.
[0026] (2) Inoculate in 50 mL LB medium according to 1% inoculum amount, and culture at 37°C until OD600 is about 0.6 (about 2-3 hours).
[0027] (3) Transfer t...
Embodiment 2
[0055] This example is the induced expression and separation and purification of the esterase of the present invention.
[0056] 1. Add 500μl recombinant bacteria (BL21 Escherichia coli) solution to a 50ml shake flask. Cultivate at 37°C for 2.5h, and stand at 15°C for 0.5h. Then add 20 μl IPTG, cold induction culture at 15°C for 24h. The fermented broth obtained from fermentation was centrifuged to obtain bacterial cells, the bacterial cells were redissolved in disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution (20mmol / L, pH7.0), crushed by an ultrasonic breaker, and the supernatant was collected by centrifugation to obtain crude enzyme liquid.
[0057] 2. Use the AKTAavant150 protein purification system to purify the crude enzyme liquid obtained in step 1 with a nickel column. The elution method is: put the four pipelines A1, A2, B1, and B2 into water, and set the flow rate of systemflow to 20ml / min. Exhaust. Then set systemflow1ml / min, flowpath (colum...
Embodiment 3
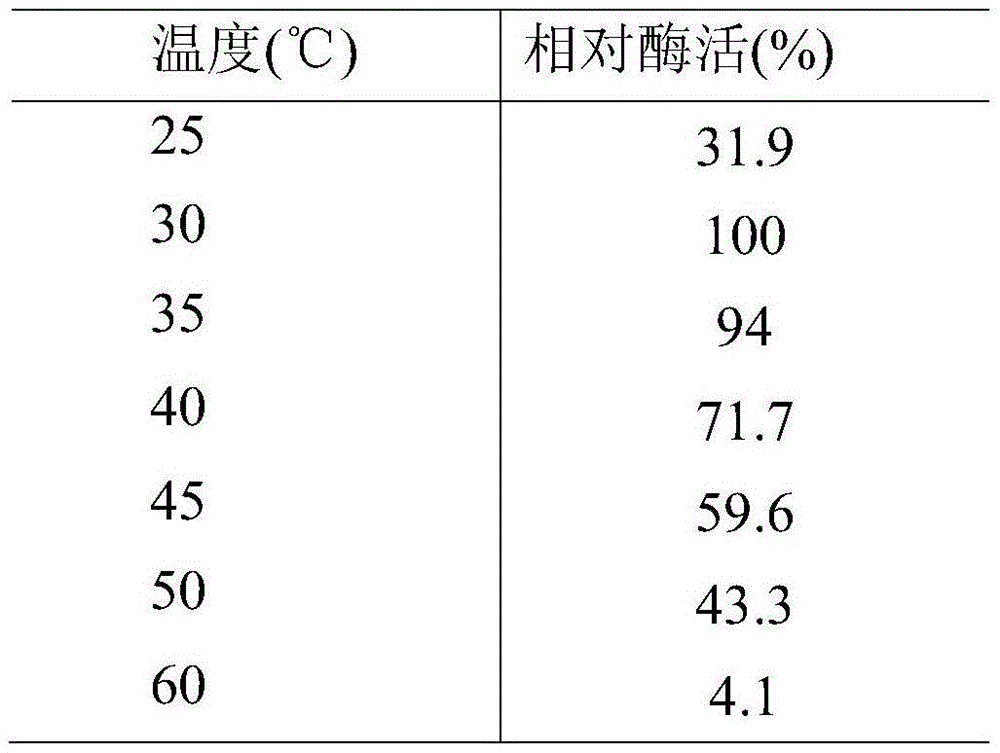
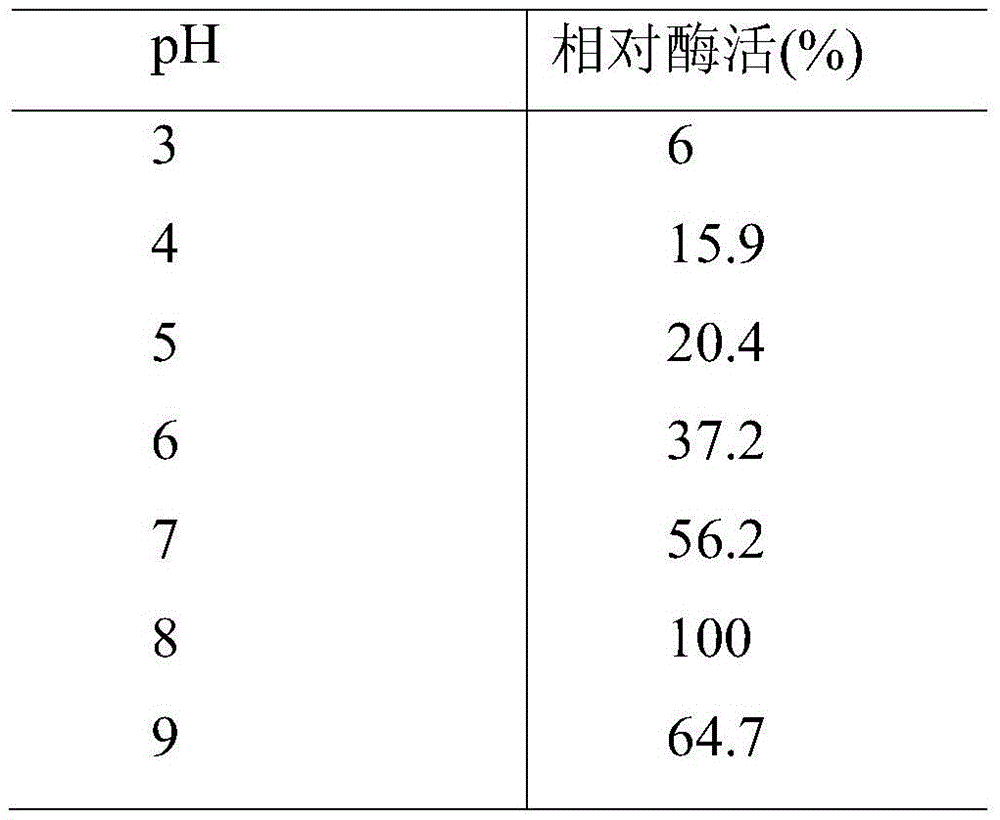
[0059] This example shows the optimum temperature and temperature stability of the esterase of the present invention. As shown in Table 1, methyl ferulate is used as a substrate, and the substrate and a phosphate buffer solution with a pH of 6.0 are tested in a water bath for 1 h at a temperature of 25-60° C. to determine the enzyme activity of the enzyme. The optimum reaction temperature is 30°C. The esterase of the present invention was treated at 30, 40, 50, and 60°C for 1 hour, and it was found that there was basically no loss of enzyme activity under the conditions of 30 and 40°C. When the temperature reached 50°C, the enzyme activity remained after 1 hour. It was 40% at the beginning, and when the temperature was higher than 60°C, the enzyme was completely inactivated after 2 hours.
[0060] The impact of table 1 temperature on esterase activity
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com