Thermal-activation delayed fluorescence material based on 4-fluorophenylacetonitrile, and preparation and application thereof
A technology of delayed fluorescence and fluorobenzene acetonitrile, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve problems such as difficulty in achieving blue light emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] Add 703 mg of carbazole and 25 mL of N,N-dimethylformamide (DMF) into a three-neck flask with a stirring bar, gradually add 5 mL of DMF solution containing 150 mg of NaH at room temperature, stir for 30 minutes, and then add 193 mg of pentafluorobenzene Acetonitrile in DMF solution 5mL, stirred for 3h.
[0034] After the reaction, pour the reaction mixture into 50 mL of ice water, filter the precipitate, use silica gel as the stationary phase, and use a mixed solution of petroleum ether and dichloromethane with a volume ratio of 1 to 3:1 as the eluent, and separate it by column chromatography to obtain Compound CyFbCz yellow solid 327mg, yield 42%.
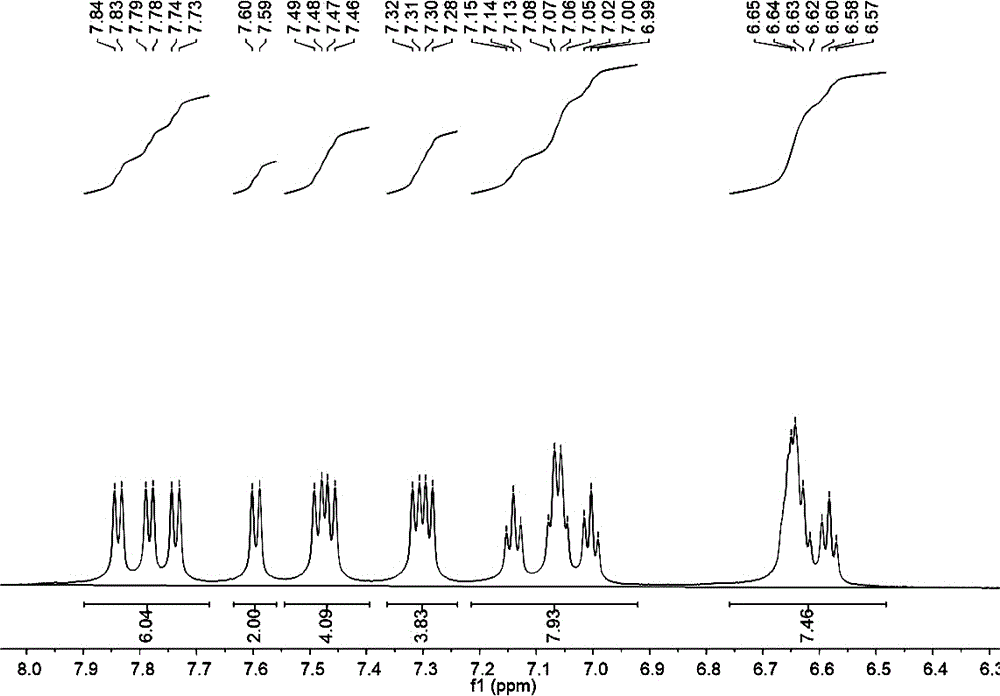
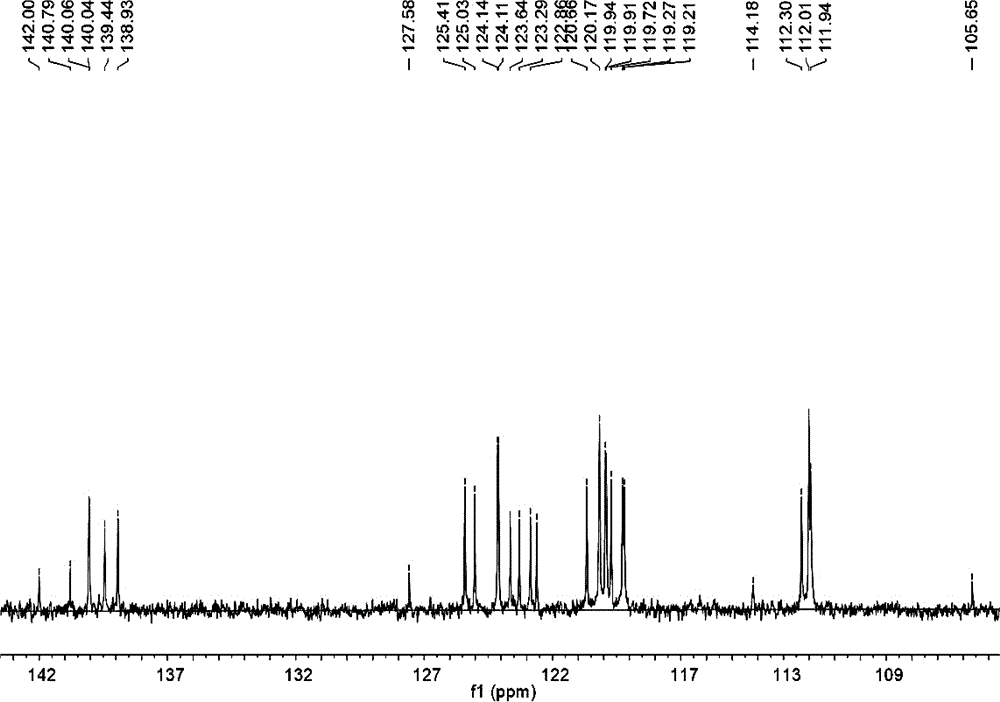
[0035] figure 1 In the H NMR spectrum, the chemical shifts at 7.84-7.59ppm, 7.49-7.28ppm, 7.15-6.99ppm, and 6.65-6.57ppm are aromatic hydrogens on the carbazole ring. Since the product is a symmetrical structure, the carbazole ring has a total of Four groups of hydrogens appeared, and the number of hydrogens i...
Embodiment 2
[0043]
[0044] Add 824 mg of phenoxazine and 30 mL of DMF into a three-necked flask with a stirring bar, gradually add 5 mL of DMF solution containing 170 mg of NaH at room temperature, stir for 40 min, then add 5 mL of DMF solution containing 193 mg of pentafluorophenylacetonitrile, and stir for 4.5 h.
[0045] After the reaction, pour the reaction mixture into 40 mL of ice water, filter the precipitate, use silica gel as the stationary phase, and use a mixed solution of petroleum ether and dichloromethane with a volume ratio of 1 to 4:1 as the eluent, and separate it by column chromatography to obtain Compound CyFbPOZ yellow solid 313mg, yield 37%.
[0046] 1 HNMR (500MHz, DMSO- d 6 )δ(ppm):7.33-7.29(m,8H),6.98(d, J =7.2Hz, 4H), 6.95-6.91(m, 4H), 6.75-6.70(m, 8H), 6.75-6.71(m, 8H).
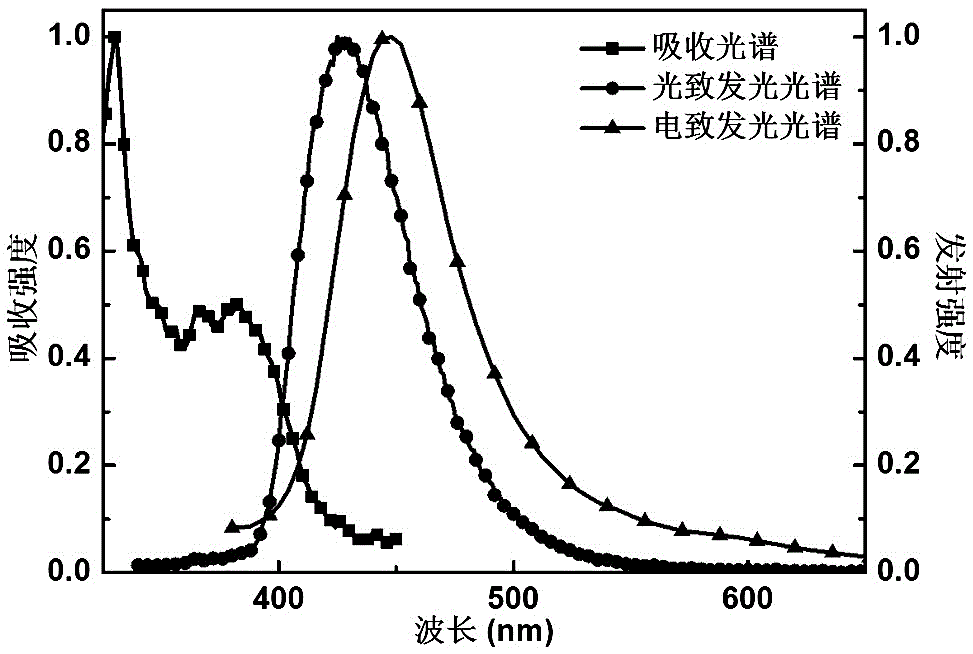
[0047] The compound CyFbPOZ prepared in this example was doped in the mCP host material by vacuum evaporation to form a thin film, and the transient fluorescence decay curve at room temp...
Embodiment 3
[0050]
[0051] Add 856 mg of phenothiazine and 35 mL of DMF into a three-neck flask with a stirring bar, gradually add 7 mL of DMF solution containing 190 mg of NaH at room temperature, stir for 1 h, then add 5 mL of DMF solution containing 193 mg of pentafluorophenylacetonitrile, and stir for 7 h.
[0052] After the reaction, pour the reaction mixture into 60 mL of ice water, filter the precipitate, use silica gel as the stationary phase, and use a mixed solution of petroleum ether and methylene chloride with a volume ratio of 1 to 2:1 as the eluent. After separation by column chromatography, Obtained compound CyFbPTZ brown yellow solid 409mg, yield 45%.
[0053] 1 HNMR (500MHz, DMSO- d 6 )δ(ppm):7.69-7.63(m,8H),7.55-7.52(m,4H),7.50-7.46(m,4H),7.41(d, J =7.2Hz, 4H), 7.36-7.33(m, 4H), 7.29-7.25(m, 8H).
[0054] The compound CyFbPTZ prepared in this example is doped into the mCP host material to make a thin film, and the transient fluorescence decay curve at room temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical bandgap | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
| Current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com