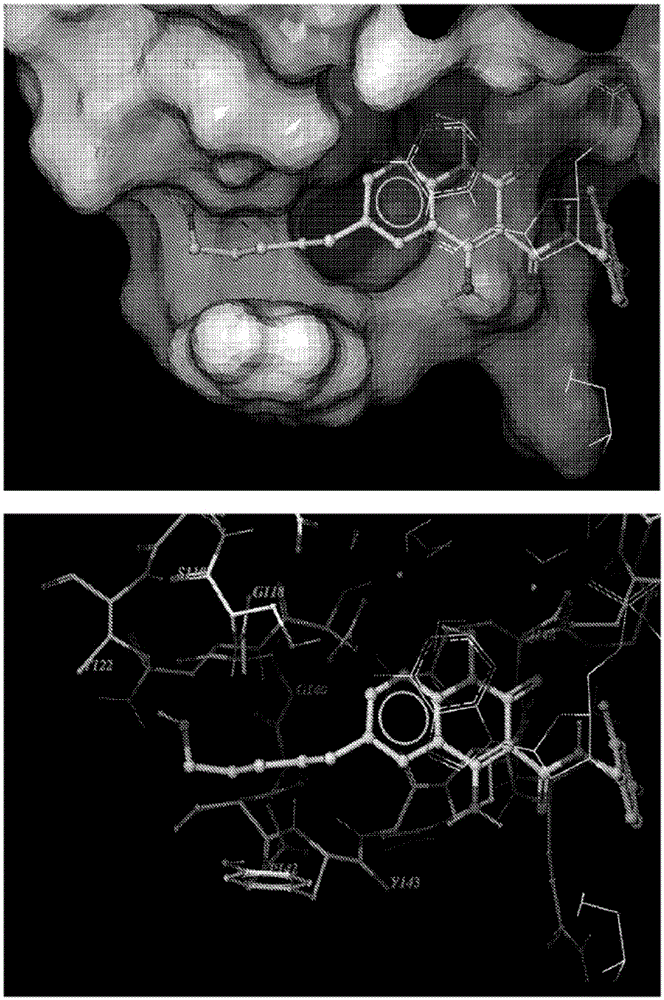
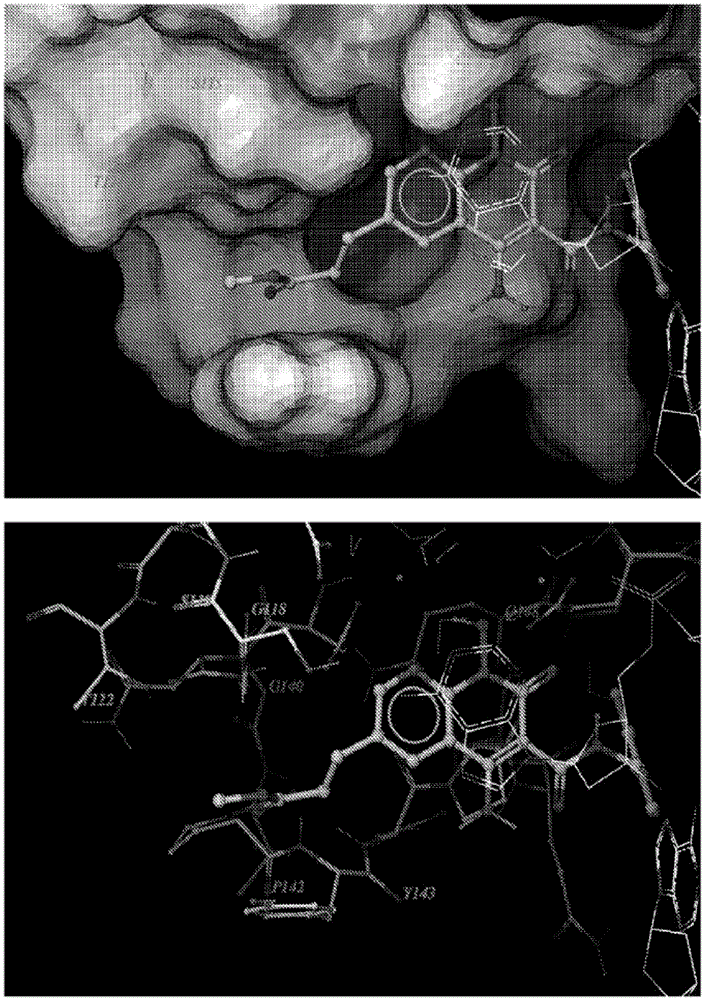
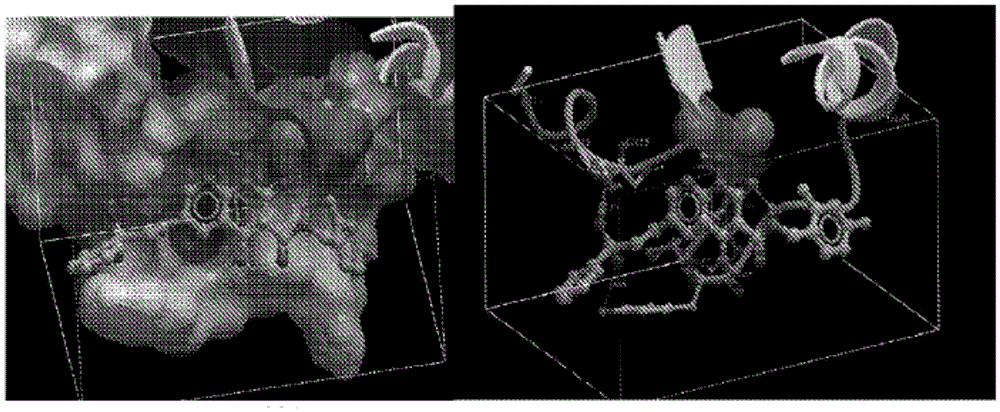
Compounds for inhibiting drug-resistant strains of HIV-1 integrase
A technology of HIV-1 and compounds, applied in the field of compounds for inhibiting drug-resistant HIV-1 integrase drug-resistant strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0174] Scheme B. Preparation of Starting Pyridine Analogs
[0175]
[0176] Scheme C. Preparation of 1-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide analogs.
[0177]
[0178] Scheme D. 1-Hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide and 2-oxo-1,2-dihydro-1,8-naphthyridine- Preparation of 3-carboxamide analogues.
[0179]
[0180] Scheme E. Preparation of 1-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide analogs.
[0181]
[0182] Scheme F. Preparation of 4-amino-1-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide analogs.
[0183]
[0184] The heck reaction: R 8 = alkenyl
[0185] Suzuki Reaction: R 8 = aryl
[0186] Sonogashira reaction: R 8 = Alkyl
[0187] Buchwald-Hartwig amination: R 8 = amine
[0188] Ullmann Response: R 8 = ether
[0189] Negishi reacts: R 8 = Alkyl
[0190] Scheme G. Preparation of 1-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamide analogs using coupling reactions.
[019...
Embodiment
[0594] Assay protocol:
[0595] In vitro integrase assay. The integrase reaction was performed by adding drug or an equal volume of 100% dimethyl sulfoxide (DMSO; used as drug solvent) to 20 nM DNA and 400 nM integrase in 50 mM morpholine propane sulfonic acid (pH 7.2), 7.5 mM MgCl 2 and 14 mM 2-mercaptoethanol in a mixture. Reactions were performed at 37°C for 2 hours and quenched by adding an equal volume of loading buffer (formamide containing 1% sodium dodecyl sulfate, 0.25% bromophenol blue, and xylene cyanol). Reaction products were separated on a 16% polyacrylamide denaturing sequencing gel. Dried gels were visualized using a Typhoon 8600 (GE Healthcare, Piscataway, NJ). Densitometric analysis was performed using ImageQuant 5.1 software from GE Healthcare. Data analysis of at least 3 independent measurements was performed using GraphPad's Prism 5.0c software (linear regression, 50% inhibitory concentration [IC 50 ] determination, standard deviation [SD]).
[059...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com