Method for coproduction of aromatic hydrocarbon and methane by direct conversion of synthesis gas
A technology for synthesis gas and methane, which is applied in chemical instruments and methods, hydrocarbon production from carbon oxides, and condensation hydrocarbon production with dehydrogenated hydrocarbons, etc., can solve the problems of low reaction pressure, reduced conversion rate, high temperature, etc. Achieve the effects of high methanation conversion rate, lower reaction temperature, and high heating efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Catalyst of the present invention can be prepared as follows:
[0057] 1)(Ni,Mo) / SiO 2 Two-step impregnation preparation of the existing isometric impregnation method for bimetallic precursor catalysts:
[0058] SiO 2 Treated in air at 200°C for 2h, then treated with Ni(NO 3 ) 2 ·6H 2 O is impregnated with an equal volume of Ni source, the active metal Ni loading is 10wt%, vacuum treatment for 1h, drying at 120°C for 12h, and calcination at 550°C for 2h to obtain Ni / SiO 2 Granular catalyst; as (NH 4 )6Mo 7 o 24 4H 2 O is impregnated with an equal volume of Mo source, the active metal Mo loading is 10wt%, vacuum treatment for 1h, drying at 120°C for 12h, and calcination at 550°C for 2h to obtain (Ni,Mo) / SiO 2 granular catalyst.
[0059] 2) Tetrapropylammonium hydroxide (TPAOH) was used as template, and Al(NO 3 ) 3 9H 2 O is the source of Al, with (Ni,Mo) / SiO 2 The Si dissolved out during the synthesis was used as the Si source; the Al(NO 3 ) 3 9H 2 O is ...
Embodiment 2
[0068] Repeat Example 1, the catalyst preparation method and reaction conditions are the same as in Example 1, except that the reaction space velocity becomes 3000ml / g.cat.h, and the reaction results are shown in Table 2 below.
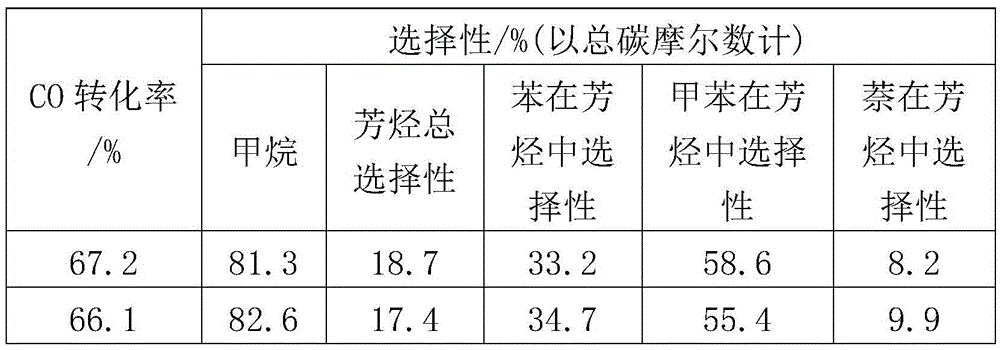
[0069] Table 2
[0070]
[0071]
[0072] The data in Table 2 show that the smaller the space velocity, the less heat released by methanation, so that the anaerobic aromatization of methane is not sufficient, so the selectivity of aromatics is not high.
Embodiment 3
[0074] Repeat Example 1, the catalyst preparation method and reaction conditions are the same as in Example 1, except that the reaction space velocity becomes 10000ml / g.cat.h, and the reaction results are shown in Table 3 below.
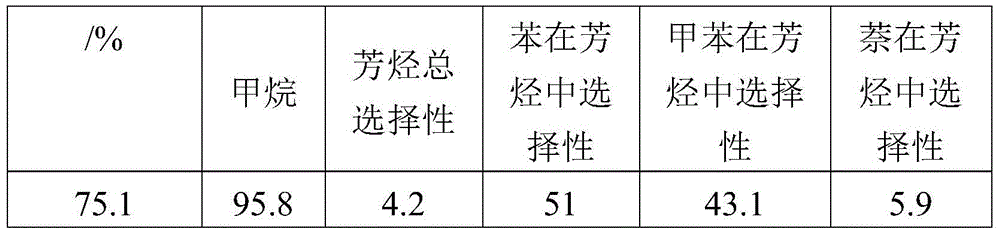
[0075] table 3
[0076]
[0077] The data in Table 3 show that the larger the space velocity, the heat released by methanation is sufficient, but the faster gas velocity makes the anaerobic aromatization of methane insufficient, and the excess gas will take away a lot of heat, so the aromatics Not very selective.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com