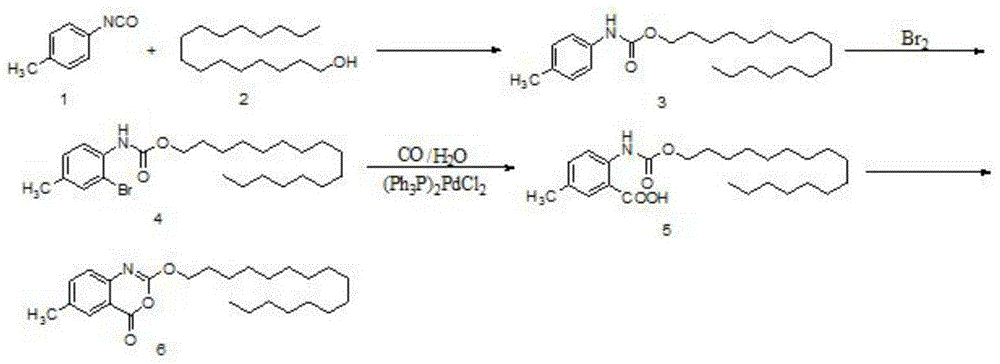
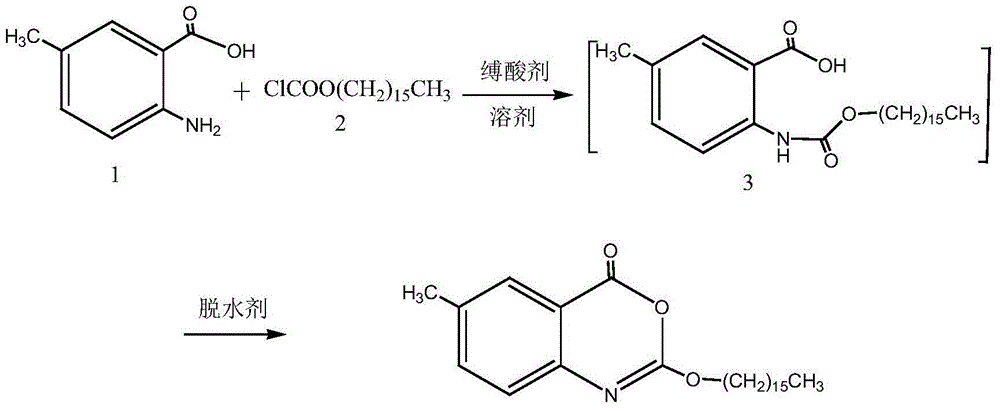
One-pot high-yielding preparation of cetilistat
A technology of new listat and process, which is applied in the field of one-pot high-yield preparation of neolistat, which can solve the problems of high equipment requirements, high safety risks, and inability to recycle, and achieve good quality, simple post-treatment, and high-quality technology. The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation one of embodiment 1 new lixistat
[0028] Add 2-amino-5-methylbenzoic acid (2.0g, 13.2mmol) and 20ml of pyridine in the reaction flask, stir and dissolve the mixture, add hexadecyl chloroformate (4.2g, 14.0mmol) dropwise under ice-bath cooling , after dropping, the ice bath was removed, and the reactant was stirred and reacted at room temperature for 2 hours, and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (2.6g, 13.6mmol) was added, After the reaction mixture was stirred at room temperature for 2 hours, it was concentrated to recover pyridine, the residue was added with 300 ml of water and stirred for 5 minutes, filtered, the solid was washed with water, and dried to obtain 5.2 g of off-white solid (98.1% yield). The crude product was recrystallized from methanol-ethyl acetate to obtain 4.6 g of white crystals (recrystallization yield 89.0%), mp76-78°C, HPLC purity 99.2%.
[0029] 1 HNMR (CDCl 3 ,400MHz): δ7.91(s,1H,Ar-H),7.51(d,J=8.0...
Embodiment 2
[0031] The preparation two of embodiment 2 new lixistat
[0032] Add 2-amino-5-methylbenzoic acid (10.0g, 66mmol) and 100ml pyridine in the reaction flask, the mixture is stirred and dissolved, and hexadecyl chloroformate (21g, 70mmol) is added dropwise under ice-bath cooling, and the dropwise After the ice bath was removed, the reactant was stirred at room temperature for 2 hours, and thionyl chloride (8.3 g, 70 mmol) was added dropwise. After the reaction mixture was stirred at room temperature for 1 hour, the pyridine solvent was recovered by concentration, and the residue was added with 500 ml of water and stirred for 10 minutes. Filter, wash the solid with water, and dry to obtain 25.7 g of a light yellow solid (97.0% yield). The crude product was recrystallized from ethyl acetate to obtain 23 g of white crystals (recrystallization yield 89.5%), mp76-78°C.
[0033] 1 HNMR (CDCl 3 ,400MHz): δ7.91(s,1H,Ar-H),7.52(d,J=8.0Hz,1H,Ar-H),7.32(d,J=8.4Hz,1H,Ar-H),4.42 (t, J=6.4...
Embodiment 3
[0034] Embodiment 3 The preparation three of new lixistat
[0035] Add 2-amino-5-methylbenzoic acid (10.0g, 66mmol) and 100ml pyridine in the reaction flask, the mixture is stirred and dissolved, and hexadecyl chloroformate (21g, 70mmol) is added dropwise under ice-bath cooling, and the dropwise After the ice bath was removed, the reactant was stirred and reacted at room temperature for 2 hours, and phosphorus oxychloride (8.2 g, 54 mmol) was added dropwise. After the reaction mixture was stirred at room temperature for 2 hours, the pyridine solvent was concentrated and recovered, and the residue was added with 600 ml of water and stirred for 10 minutes. Filter, wash the solid with water, and dry to obtain 25.3 g of white solid (95.5% yield). The crude product was recrystallized with 95% ethanol to obtain 22.5 g of white crystals (recrystallization yield 91.8%), mp76-78°C.
[0036] 1 HNMR (CDCl 3 ,400MHz): δ7.91(s,1H,Ar-H),7.51(d,J=8.0Hz,1H,Ar-H),7.32(d,J=8.4Hz,1H,Ar-H),4.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com