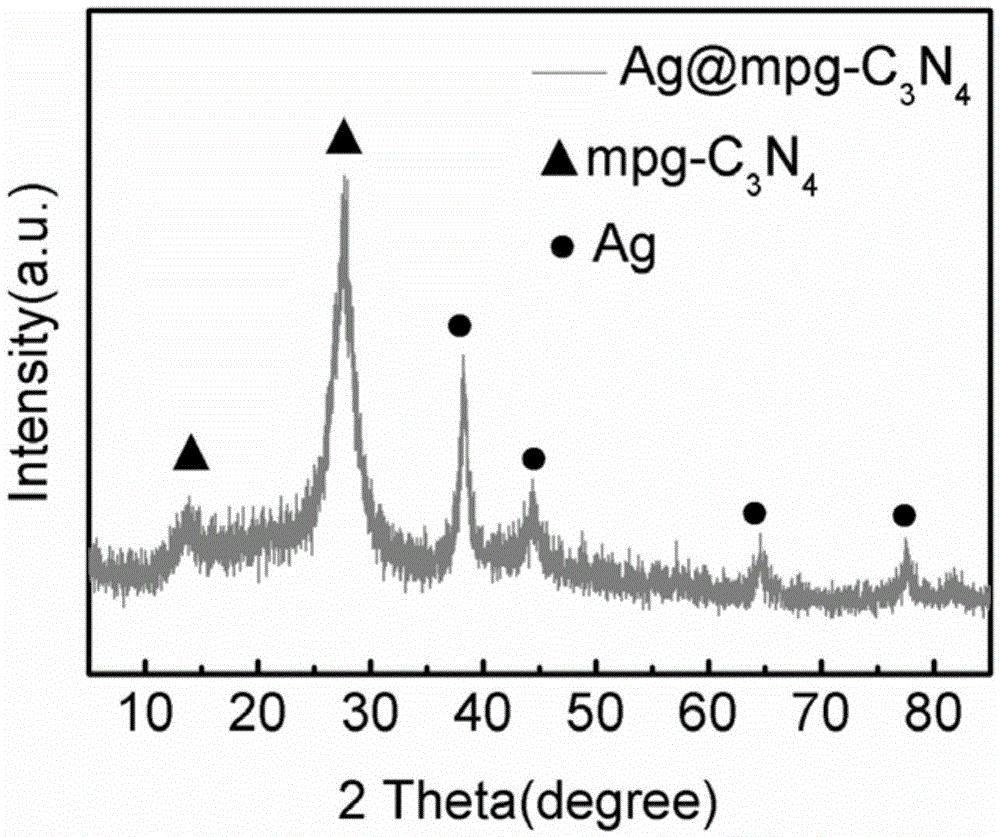
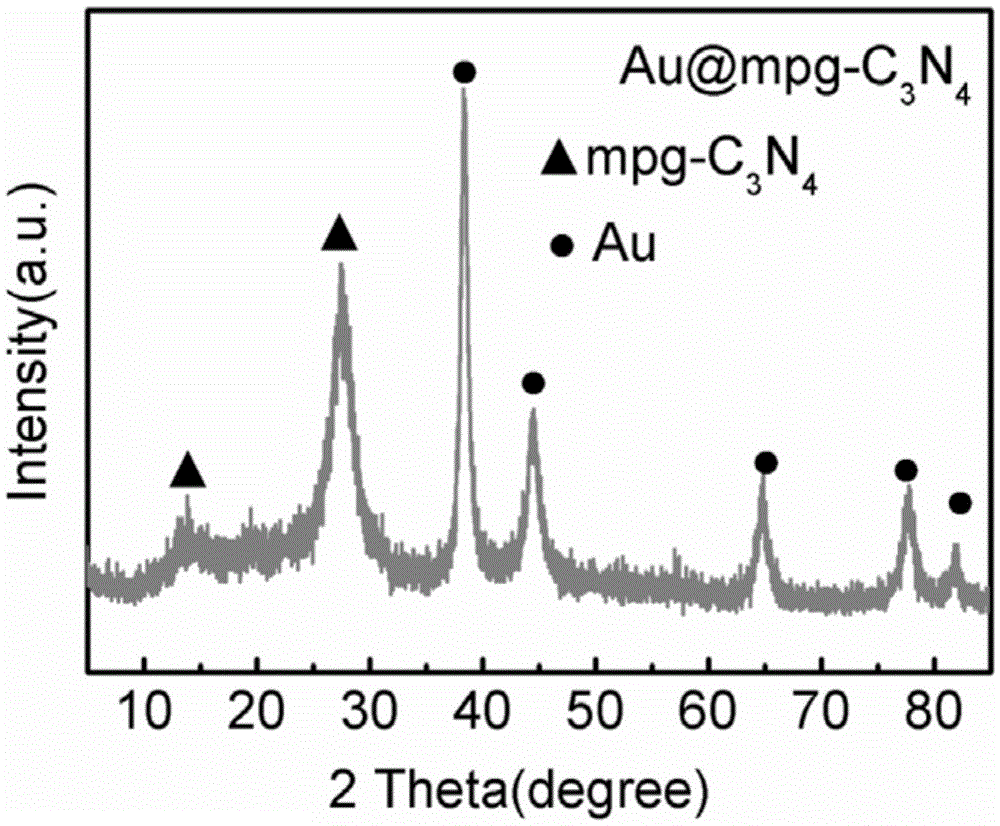
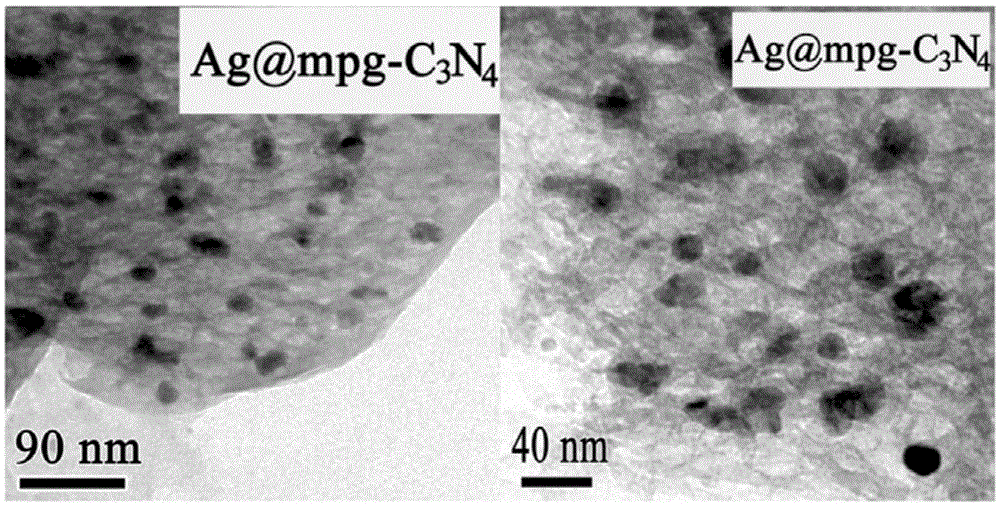
Loaded graphite phase carbonized nitrogen composite material as well as preparation method and application thereof
A composite material, graphite phase technology, applied in a new composite nanocatalyst and its application in the reduction of p-nitrophenol, can solve the problem of difficulty in obtaining highly dispersed and stable precious metal nanoparticles and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Dissolve 2 g of melamine in 80 ml of deionized water, and heat to 80° C. until completely dissolved to form a uniform and transparent solution A.
[0032] 2) Add 1.5ml of concentrated hydrochloric acid with a concentration of 30wt% into solution A, stir for 30 minutes and mix evenly to form mixed solution B.
[0033] 3) Stir the above mixed solution at a constant temperature of 80° C. until all the white solids are precipitated, and grind it into powder A.
[0034] 4) Place the obtained white powder sample A in a 50ml crucible, place it in a muffle furnace and heat it to 500°C, heat and condense for 2 hours, place it at 80°C for a certain period of time, take it out, and cool it down to room temperature naturally After that, the light yellow product obtained is porous graphite phase nitrogen carbide.
Embodiment 2
[0036] (1) Dissolve 2 g of melamine in 80 ml of deionized water, and heat to 80° C. until completely dissolved to form a uniform and transparent solution A.
[0037] (2) Add 1.5ml of concentrated hydrochloric acid with a concentration of 30wt% into solution A, stir for 30 minutes and mix evenly to form mixed solution B.
[0038] (3) Stir the above mixed solution at a constant temperature of 80° C. until all the white solid is precipitated, and grind it into powder A.
[0039] (4) Place the obtained white powder sample A in a 50ml crucible, place it in a muffle furnace and heat it to 500°C, heat and condense for 2 hours, place it at 80°C for a certain period of time, take it out, and cool it naturally to After room temperature, the light yellow product is porous graphite phase nitrogen carbide.
[0040] (5) With the porous graphite phase nitrogen carbide (mpg-C obtained in the above steps 3 N 4 ) as a template, 100mg of mpg-C 3 N 4 Add it into a mixed solution of 20ml of eth...
Embodiment 3
[0048] (1) Dissolve 2 g of melamine in 80 ml of deionized water, and heat to 80° C. until completely dissolved to form a uniform and transparent solution A.
[0049] (2) Add 1.5ml of concentrated hydrochloric acid with a concentration of 30wt% into solution A, stir for 30 minutes and mix evenly to form mixed solution B.
[0050] (3) Stir the above mixed solution at a constant temperature of 80° C. until all the white solid is precipitated, and grind it into powder A.
[0051] (4) Place the obtained white powder sample A in a 50ml crucible, place it in a muffle furnace and heat it to 500°C, heat and condense for 2 hours, place it at 80°C for a certain period of time, take it out, and cool it naturally to After room temperature, the light yellow product is porous graphite phase nitrogen carbide.
[0052] (5) With the porous graphite phase nitrogen carbide (mpg-C obtained in the above steps 3 N 4 ) as a template, 100mg of mpg-C 3 N 4 Add it to 20ml of isopropanol and water m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com