Supported amorphous state nickel phosphide catalyst used for light paraffin isomerization and preparation method and using method thereof
A technology of light alkanes and catalysts, which is applied in the field of catalysts for isomerization of light alkanes, can solve the problems of high operating temperature, achieve the effects of avoiding pollution, good stability, and reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
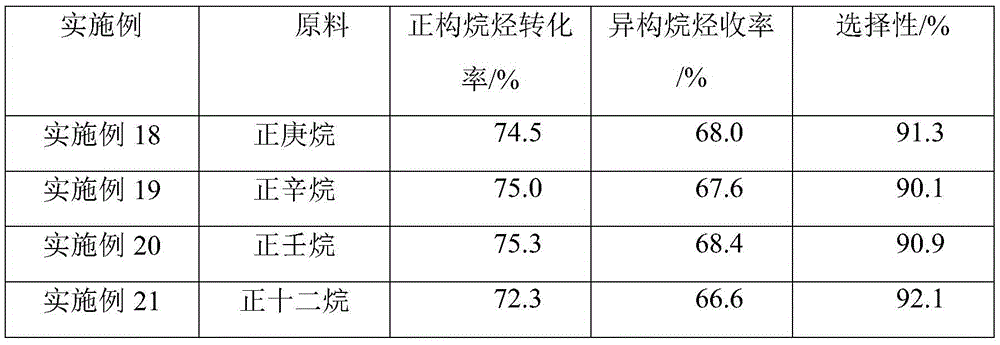
Examples
Embodiment 1
[0034] The catalyst for the isomerization of light alkanes described in this example comprises a carrier and nickel phosphide accounting for 5 wt% of the carrier weight, wherein the carrier is composed of alumina and Hβ molecular sieves, and the alumina and The weight ratio of Hβ molecular sieve is 1:3.
[0035] The preparation method of the catalyst for light alkane isomerization described in this embodiment is:
[0036] Calcinate the Hβ molecular sieve at 500°C for 4 hours, then mix 1 weight part of alumina powder with 3 weight parts of the roasted Hβ molecular sieve, add 0.2 weight part of nickel phosphide, mix well and extrude into strips, 50°C After drying, it was ground into particles with a particle size of 0.45 mm, and then the particles were dried at 300° C. for 2 hours under a nitrogen atmosphere to prepare catalyst A1.
[0037]Catalyst A1 prepared in this example was prepared at a reaction temperature of 280°C, a reaction pressure of 1.0 MPa, a hydrogen-to-oil mola...
Embodiment 2
[0039] The catalyst for the isomerization of light alkanes described in this example comprises a carrier and nickel phosphide accounting for 3wt% of the carrier weight, wherein the carrier is composed of alumina and Hβ molecular sieves, and the alumina and The weight ratio of Hβ molecular sieve is 1:9.
[0040] The preparation method of the catalyst for light alkane isomerization described in this embodiment is:
[0041] Calcinate the Hβ molecular sieve at 500°C for 4 hours, then mix 1 weight part of alumina powder with 9 weight parts of the roasted Hβ molecular sieve, add 0.3 weight part of nickel phosphide, mix evenly and extrude into strips at 50°C After drying, it was ground into particles with a particle size of 0.45 mm, and then the particles were calcined at 100° C. under a nitrogen atmosphere for 2 hours to prepare catalyst A2.
[0042] Catalyst A2 prepared in this example was prepared at a reaction temperature of 230°C, a reaction pressure of 2.0 MPa, a hydrogen-to-o...
Embodiment 3
[0044] The catalyst for the isomerization of light alkanes described in this example comprises a carrier and nickel phosphide accounting for 5 wt% of the carrier weight, wherein the carrier is composed of alumina and Hβ molecular sieves, and the alumina and The weight ratio of Hβ molecular sieve is 9:1.
[0045] The preparation method of the catalyst for light alkane isomerization described in this embodiment is:
[0046] Calcinate the Hβ molecular sieve at 500°C for 4 hours, then mix 9 parts by weight of alumina powder with 1 part by weight of the calcined Hβ molecular sieve, add 0.5 parts by weight of nickel phosphide, mix well and then extrude into strips at 50°C After drying, it was ground into particles with a particle size of 0.45 mm, and then the particles were calcined at 100° C. under a nitrogen atmosphere for 2 hours to prepare catalyst A3.
[0047] The catalyst A3 prepared in this example was prepared at a reaction temperature of 230°C; a reaction pressure of 2.0MP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com