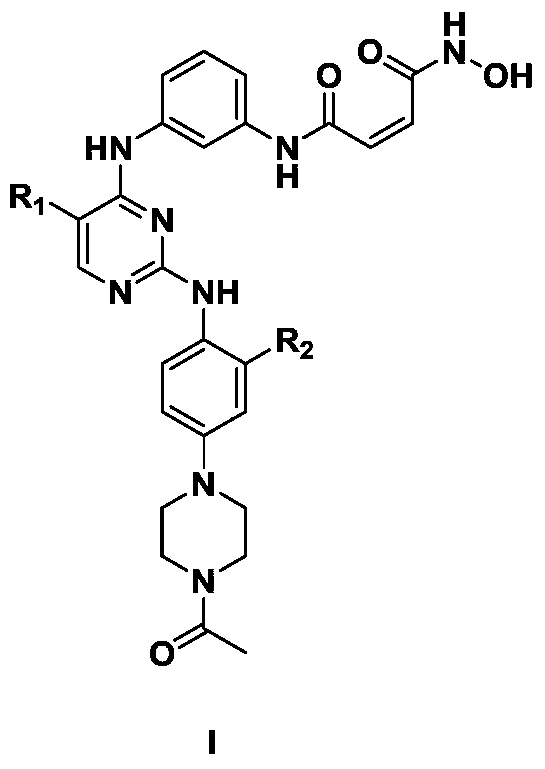
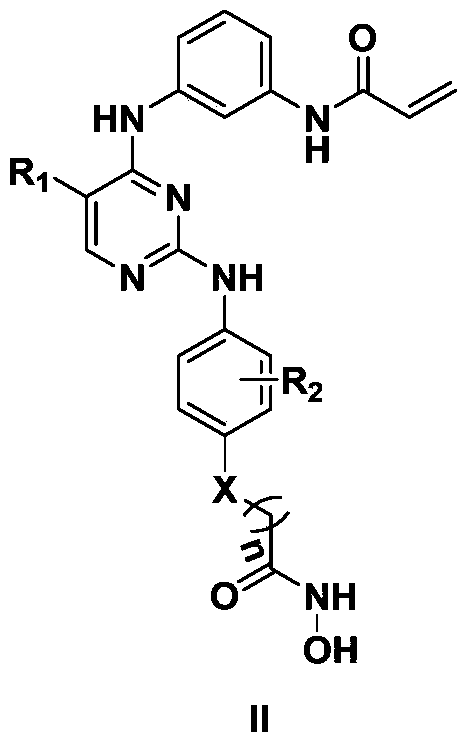
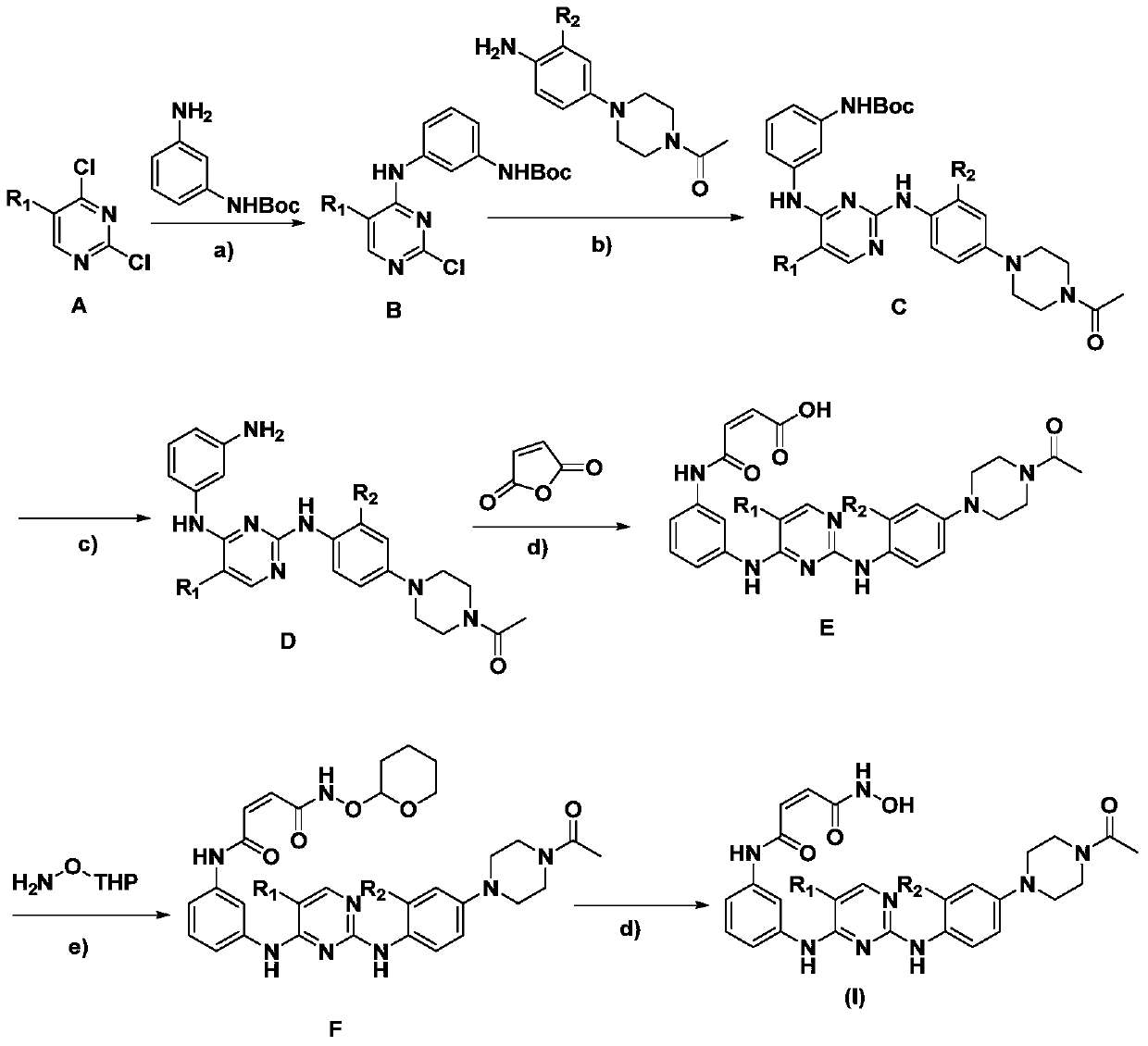
2,4-diarylaminopyrimidine derivatives containing hydroxamic acid fragments and their preparation and application
A technology of diarylaminopyrimidine and hydroxamic acid, applied in the field of 2,4-diarylaminopyrimidine derivatives and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1N 1 -(3-((2-((4-(4-acetyl-piperazin-1-yl)-2-methoxyphenyl)amino)-5-chloropyrimidin-4-yl)amino)phenyl )-N 4 -Hydroxymaleamide hydrochloride
[0064]
[0065] Reagents and reaction conditions: a) N,N-dimethylformamide, 50°C, 3 hours; b) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 1 -Hydroxybenzotriazole, N,N-dimethylformamide:dichloromethane=1:2, 45°C, 5 hours; c) 1M hydrochloric acid ether solution, 0°C, 30 minutes to 1 hour.
[0066] Step 1: (Z)-4-((2((2-((4-(4-acetylpiperazinyl)-2-methoxy)amino)-5-chloropyrimidine)amino)phenyl)amino) - Preparation of 4-oxo-2-butenoic acid
[0067] Starting material 1: 2-((2-methoxy-4-(N 4 -Acetylpiperazinyl)-aminophenyl)-4-(3-amino)aminophenyl)-5-chloropyrimidine was prepared according to the method of Nature, 2009, 462, 1070-1074.
[0068] 2-((2-methoxy-4-(N 4 -Acetylpiperazinyl)-aminophenyl)-4-(3-amino)aminophenyl)-5-chloropyrimidine (1mmol) and maleic anhydride (1.2mmol) were dissolved in 15mL dic...
Embodiment 2
[0076] Example 2: N 1 -(3-((2-((4-(4-acetyl-piperazin-1-yl)-phenyl)amino)-5-chloropyrimidin-4-yl)amino)phenyl)-N 4 -Hydroxymaleamide hydrochloride
[0077] With reference to the method of Example 1, only (Z)-4-((2((2-((4-(4-acetylpiperazinyl)-2-methoxy)amino)-5- Chloropyrimidine)amino)phenyl)amino)-4-oxo-2-butenoic acid was replaced by (Z)-4-((2((2-((4-(4-acetylpiperazinyl)amino) -5-chloropyrimidine) amino) phenyl) amino) -4-oxo-2-butenoic acid, the 2-((2-methoxy-4-(N 4 -Acetylpiperazinyl)-aminophenyl)-4-(3-amino)aminophenyl)-5-chloropyrimidine was replaced by 2-((4-(N 4 -Acetylpiperazinyl)-aminophenyl)-4-(3-amino)aminophenyl)-5-chloropyrimidine, the N in step 3 1 -(3-((2-((4-(4-acetylpiperazin-1-yl)-2-methoxyphenyl)amino)-5-pyrimidin-4-yl)amino)phenyl)-N 4 -((tetrahydro-2H-pyran-2-yl)oxo)maleic acid diamine replaced by N 1 -(3-((2-((4-(4-acetylpiperazin-1-yl)phenyl)amino)-5-pyrimidin-4-yl)amino)phenyl)-N 4 -((tetrahydro-2H-pyran-2-yl)oxo)maleic acid diamine.
[0078] ...
Embodiment 3
[0079] Example 3: 6-(4-(4-((4-((3-acryloyl-aminophenyl)amino)-5-chloropyrimidin-2-yl)amino)-phenyl)piperazine-1- base)-N-hydroxyacetamide hydrochloride
[0080]
[0081] Reagents and reaction conditions: 1) sec-butanol, reflux, 4 hours; 3) trifluoroacetic acid, dichloromethane, 1 hour; 4) acryloyl chloride, dichloromethane, -5-0 ° C, 30 minutes to 1 hour; 5) Tetrahydrofuran: water = 1:1, 1 hour; 6) Dichloromethane: N,N-dimethylformyl = 2:1, 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride, 1-hydroxybenzotriazole, 45°C, 5 hours; 7) 1M hydrochloric acid ether solution, 0°C, 30 minutes to 1 hour.
[0082] Step 1: 2-((2-Methoxy-4-(N 4 Preparation of -acetylpiperazinyl)-aminophenyl)-4-(3-amino)aminophenyl)-3-chloropyrimidine.
[0083] Raw material 1: tert-butyl 4-((2,5-dichloro-4-pyrimidinyl)amino)phenyl)carbamate was prepared according to the method of Cancerdiacovery, 2013, 3, 1404-1415.
[0084] 4-((2,5-dichloro-4-pyrimidinyl)amino)phenyl)carbamate tert-buty...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com