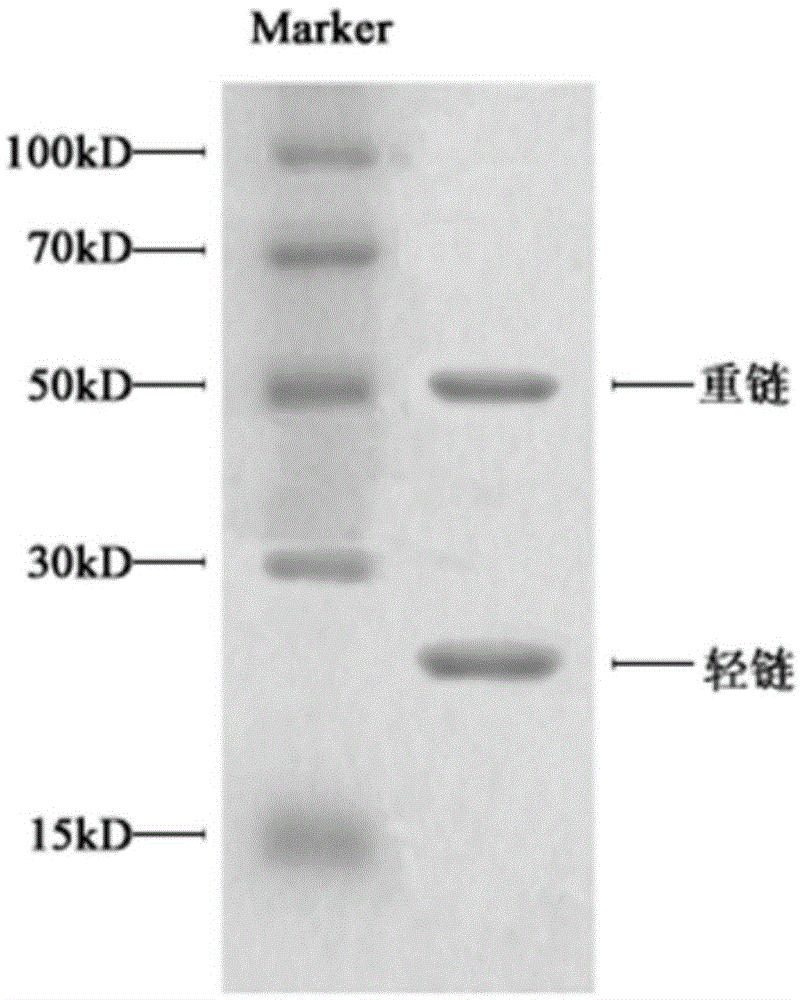
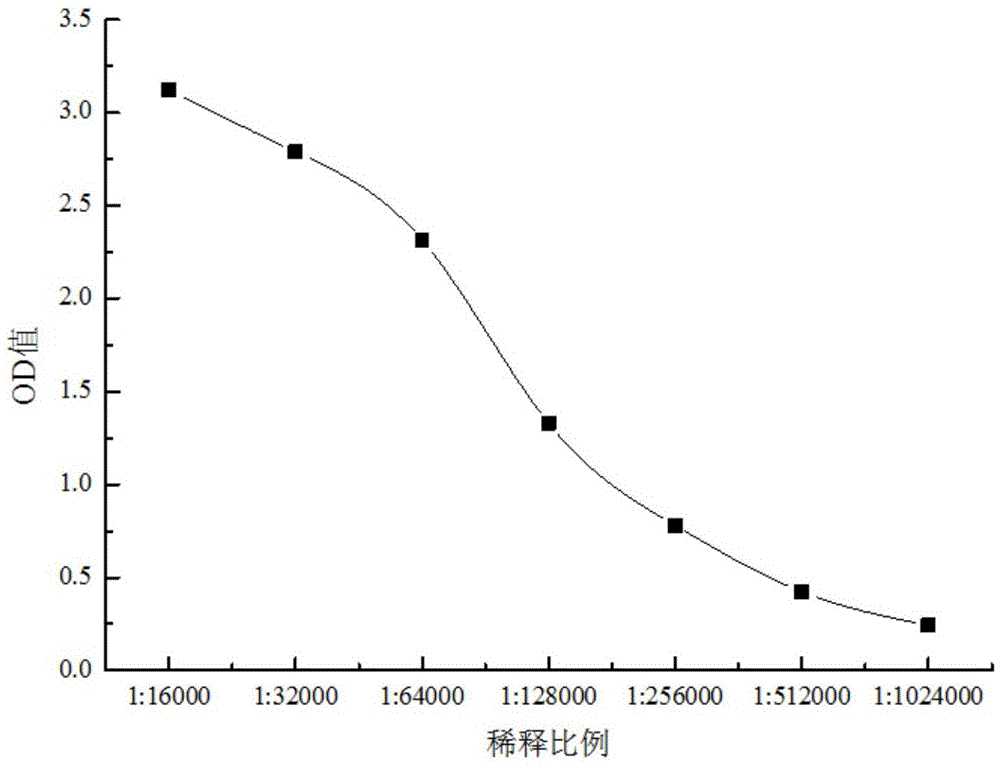
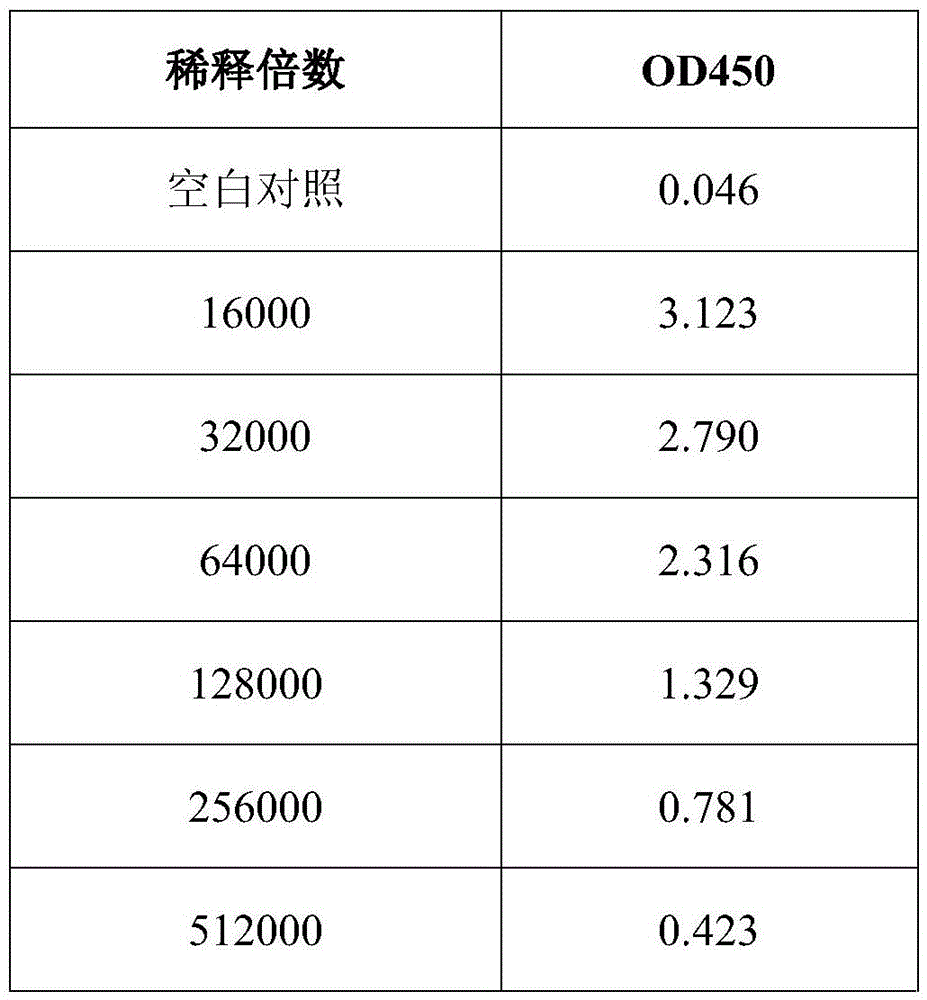
Polyclonal antibody of GXM (glucuronoxylomannan) of Cryptococcus neoformans and preparation method of polyclonal antibody
A technology of Cryptococcus neoformans and polyclonal antibodies, which is applied in antifungal/algae/lichen immunoglobulins, measuring devices, instruments, etc., can solve the problems of unguaranteed purity and specificity of antigens, poor immunogenicity, and poor properties of polyclonal antibodies. Stability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: the preparation of the polyclonal antibody of the anti-Cryptococcus neoformans capsular polysaccharide GXM antigen (hereinafter referred to as GXM antigen) of preliminary purification
[0076] 1. The inactivated Cryptococcus neoformans (ATCC, strain number: 208821) was crushed by repeated freezing and thawing and ultrasonic crushing.
[0077] The specific method is:
[0078] (1) Formaldehyde solution was added to the liquid medium to make the final concentration of formaldehyde 3.7%, and placed at 4°C for 24 hours to inactivate the spores.
[0079] (2) Centrifuge the inactivated Cryptococcus neoformans at 4000 rpm for 10 min at 4°C, and remove the supernatant.
[0080] (3) Add liquid nitrogen and grind with a sterilized mortar for 3-5 times, add water, and use probe-type ultrasonic crushing for 30 minutes under ice bath.
[0081] (4) Carry out BCA protein quantification and phenol sulfate method sugar quantification.
[0082] 2. Immunized animals
[00...
Embodiment 2
[0090] Example 2: Preparation of Cryptococcus neoformans Capsular Polysaccharide GXM Immunoaffinity Chromatography Column
[0091] (1) Dissolve the GXM antigen in carbonate buffer, bind 4 mg of GXM antigen per milliliter of wet protein A microbeads, mix the GXM antigen and protein A microbeads to form a thin homogenate, and mix in a total of 10 mL of carbon dioxide Add about 2mL of protein A microbeads to salt buffer solution, incubate at room temperature for 1h, and shake gently to mix.
[0092](2) Wash the homogenate twice with 5-15 times the volume of 0.2mol / L sodium borate (pH8.0-9.5), centrifuge at 3000g for 2min each time, or centrifuge at 10000g for 30s to obtain GXM antigen-protein A microbeads mixture.
[0093] (3) Resuspend the mixture of GXM antigen-protein A microbeads with 5-15 times the volume of 0.2mol / L sodium borate (pH8.0-9.5), take a sample equivalent to 10mL of wet protein A microbeads, add enough Add an appropriate amount of dimethylpimelate (solid) to t...
Embodiment 3
[0096] Example 3: Immunoaffinity purification of polyclonal antibody against Cryptococcus neoformans capsular polysaccharide GXM antigen
[0097] (1) Wash the column with 10-25 times the column bed volume of pre-elution buffer.
[0098] (2) Load the initially purified polyclonal antibody against the capsular polysaccharide GXM antigen of Cryptococcus neoformans, and let the sample flow through the chromatographic column at a flow rate of about 1 mL / h per milliliter of column volume, and control the flow rate with a peristaltic pump.
[0099] (3) Wash the column with 10-25 times the column bed volume of binding buffer.
[0100] (4) Wash the column with 10-25 times the column bed volume of pre-elution buffer.
[0101] (5) Using the segmented elution method, continuously pass the elution buffer of 0.4-0.8 times the column bed volume through the chromatography column, and collect each component in separate tubes.
[0102] (6) Detect the antibody content of each tube, and combine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com