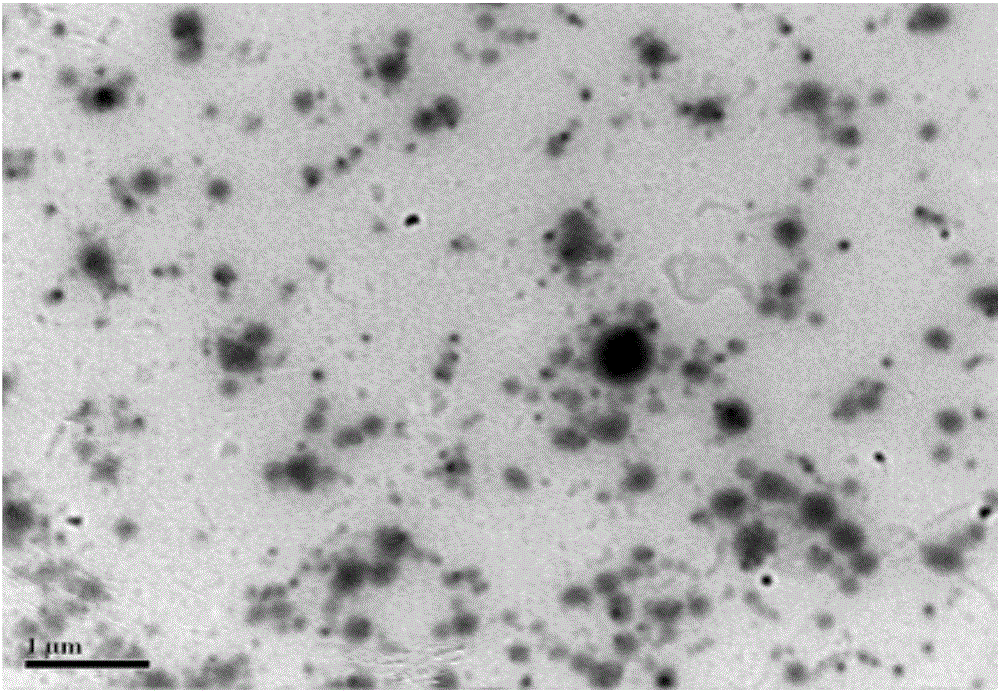
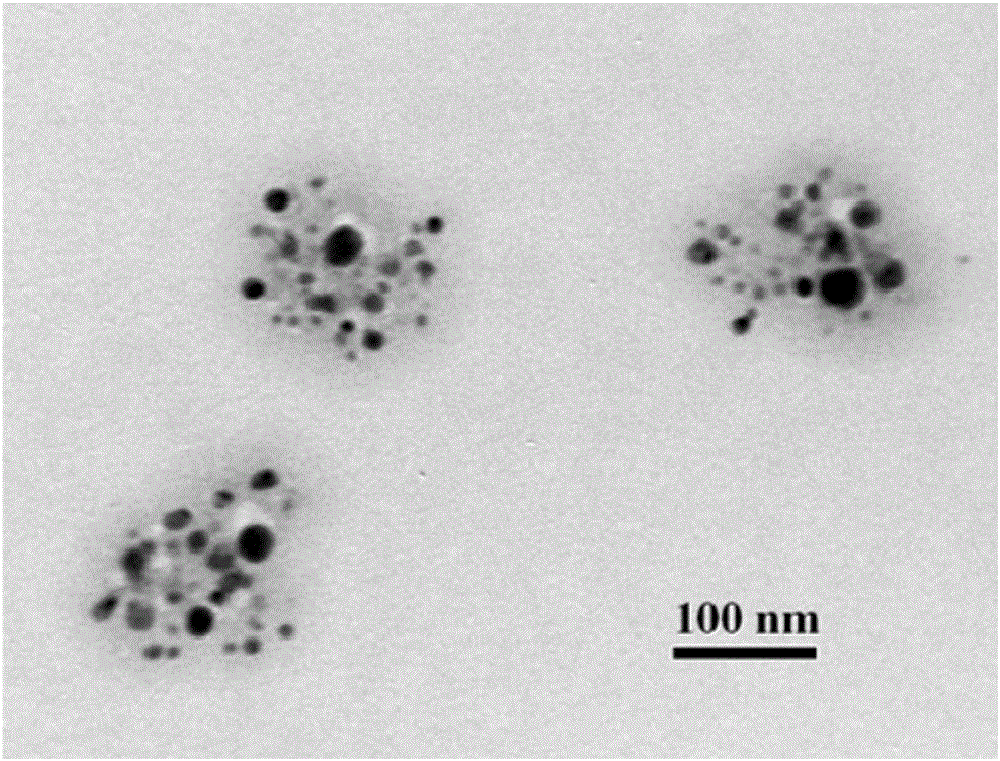
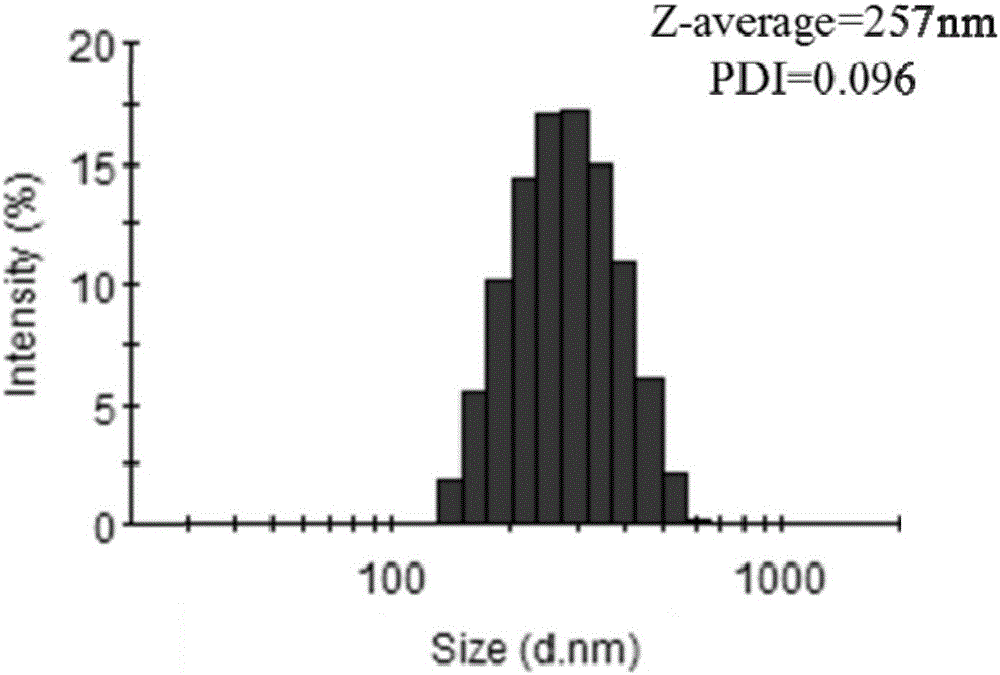
Mitochondria-targeted polysaccharide nano preparation and preparing method thereof
A nano-preparation, mitochondrial technology, applied in non-active ingredients medical preparations, medical preparations containing active ingredients, glycopeptide ingredients, etc., can solve problems such as drug leakage, achieve increased toxicity, reasonable preparation method design, and difficult The effect of leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Dissolve 40mg of triphenylphosphine TPP in 15ml of ultrapure water, 19mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide EDC, and 12mg of N-hydroxysuccinimide NHS in 1ml of ultrapure water Add the pure water to the TPP solution drop by drop, adjust the pH to 5.5, and stir the solution on a magnetic stirrer for 40 minutes. Dissolve 22 mg n-BOC-1,6-diaminohexane hydrochloride in 5 ml of water, add dropwise to the activated TPP solution, adjust the pH to 7.5, react and stir for 4 hours, and freeze-dried TPP-C 6 -BOC was dissolved in 20ml of methanol, 2ml of trifluoroacetic acid was added, the reaction was stirred for 1 hour, transferred to an eggplant-shaped bottle, evaporated to dryness by rotary evaporation, a yellow oily substance appeared, and saturated NaHCO was added 3 solution, extracted with chloroform, combined the chloroform layers, and evaporated to dryness to obtain TPP-C 6 -NH 2 .
[0042] Dissolve 40mg of hyaluronic acid HA (molecular weight 150000Da) in ...
Embodiment 2
[0045] Dissolve 40mg of triphenylphosphine TPP in 15ml of ultrapure water, 19mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide EDC, and 12mg of N-hydroxysuccinimide NHS in 1ml of ultrapure water Add the pure water to the TPP solution drop by drop, adjust the pH to 5.5, and stir the solution on a magnetic stirrer for 40 minutes. Dissolve 22 mg n-BOC-1,6-diaminohexane hydrochloride in 5 ml of water, add dropwise to the activated TPP solution, adjust the pH to 7.5, react and stir for 4 hours, and freeze-dried TPP-C 6 -BOC was dissolved in 20ml of methanol, 2ml of trifluoroacetic acid was added, the reaction was stirred for 1 hour, transferred to an eggplant-shaped bottle, evaporated to dryness by rotary evaporation, a yellow oily substance appeared, and saturated NaHCO was added 3 solution, extracted with chloroform, combined the chloroform layers, and evaporated to dryness to obtain TPP-C 6 -NH 2 .
[0046] Dissolve 40mg of hyaluronic acid HA (molecular weight 150000Da) in ...
Embodiment 3
[0049] Dissolve 40mg of triphenylphosphine TPP in 15ml of ultrapure water, 19mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide EDC, and 12mg of N-hydroxysuccinimide NHS in 1ml of ultrapure water Add the pure water to the TPP solution drop by drop, adjust the pH to 5.5, and stir the solution on a magnetic stirrer for 40 minutes. Dissolve 22 mg of n-BOC-1,6-diaminohexane hydrochloride in 5 ml of water, add dropwise to the activated TPP solution, adjust the pH to 7.5, stir the reaction for 4 hours, and freeze-dry. TPP-C after lyophilization 6 -BOC was dissolved in 20ml of methanol, 2ml of trifluoroacetic acid was added, the reaction was stirred for 1 hour, transferred to an eggplant-shaped bottle, evaporated to dryness by rotary evaporation, a yellow oily substance appeared, and saturated NaHCO was added 3 solution, extracted with chloroform, combined the chloroform layers, and evaporated to dryness to obtain TPP-C 6 -NH 2 .
[0050] Dissolve 40mg of hyaluronic acid HA (mol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com