Application of metal-free hydrogenation catalyst in catalyzing hydrogenation reaction of nitrobenzene and derivatives thereof
A technology of nitrobenzene derivatives and hydrogenation catalysts, which is applied in the preparation of organic compounds, catalysts for physical/chemical processes, preparation of amino compounds, etc., can solve the problems of high catalyst cost, metal loss, metal dissolution loss, etc. The method is simple, the production cost is low, and the reaction is easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
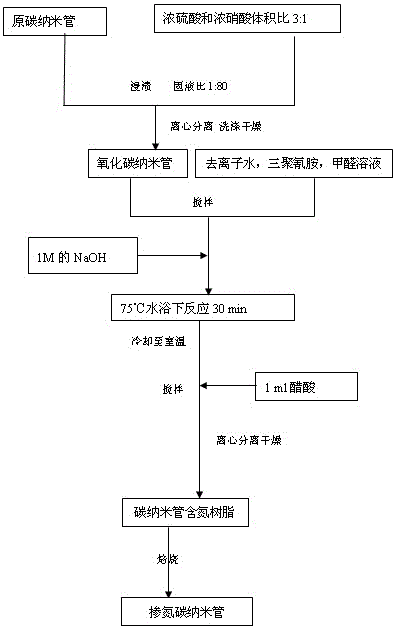
[0041] A method for preparing a metal-free hydrogenation catalyst, using melamine as a nitrogen source to prepare nitrogen-doped carbon nanotubes by means of an impregnation method, specifically comprising the following steps:
[0042] (1) Oxidation treatment of carbon nanotubes: carbon nanotubes are acidified by adding a mixed acid of concentrated sulfuric acid and concentrated nitric acid with a volume ratio of 3:1 at a solid-to-liquid ratio of 1:80 for acidification for 12 hours, centrifuged and washed, and dried at 80°C for 12 hours Obtain carbon nanotubes.
[0043] (2) Take an appropriate amount of the above-mentioned oxidized carbon nanotubes, mix them with melamine at a mass ratio of 1:1, add deionized water, and add an appropriate amount of formaldehyde twice the mass of melamine, and immerse for 12 hours;
[0044] (3) Add 1mol / L NaOH dropwise to the above solution to adjust the pH to about 10;
[0045] (4) Raise the temperature to 75°C for 30 minutes;
[0046] (5) A...
Embodiment 2
[0050] A method for preparing a metal-free hydrogenation catalyst, using melamine as a nitrogen source to prepare nitrogen-doped carbon nanotubes by means of an impregnation method, specifically comprising the following steps:
[0051] (1) Oxidation treatment of carbon nanotubes: carbon nanotubes were acidified by adding a mixed acid of concentrated sulfuric acid and concentrated nitric acid with a volume ratio of 3:1 at a solid-to-liquid ratio of 1:60 for 15 hours, centrifuged and washed, and dried at 100°C for 12 hours Obtain carbon nanotubes.
[0052] (2) Take an appropriate amount of the above-mentioned oxidized carbon nanotubes, mix them with melamine at a mass ratio of 1:1.1, add deionized water, and add an appropriate amount of formaldehyde three times the mass of melamine, and immerse for 10 hours;
[0053] (3) Add 0.5mol / L KOH dropwise to the above solution to adjust the pH to about 11;
[0054] (4) Raise the temperature to 70°C for 60 minutes;
[0055] (5) After co...
Embodiment 3
[0059] A method for preparing a metal-free hydrogenation catalyst, using melamine as a nitrogen source to prepare nitrogen-doped carbon nanotubes by means of an impregnation method, specifically comprising the following steps:
[0060] (1) Oxidation treatment of carbon nanotubes: carbon nanotubes are acidified by adding a mixed acid of concentrated sulfuric acid and concentrated nitric acid with a volume ratio of 3:1 at a solid-to-liquid ratio of 1:120 for acidification for 8 hours, centrifuged and washed, and dried at 80°C for 12 hours Obtain carbon nanotubes.
[0061] (2) Take an appropriate amount of the above-mentioned oxidized carbon nanotubes, mix them with melamine at a mass ratio of 1:1.2, add deionized water, and add an appropriate amount of formaldehyde twice the mass of melamine, and immerse for 20 hours;
[0062] (3) Add 2mol / L NaCO dropwise to the above solution 3 , adjust the pH to about 12;
[0063] (4) Raise the temperature to 80°C for 30 minutes;
[0064] (5)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com