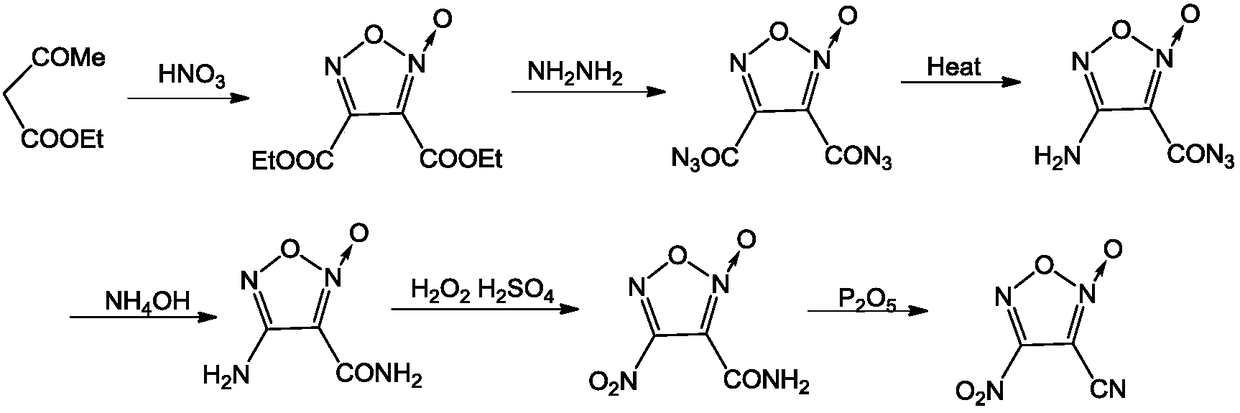
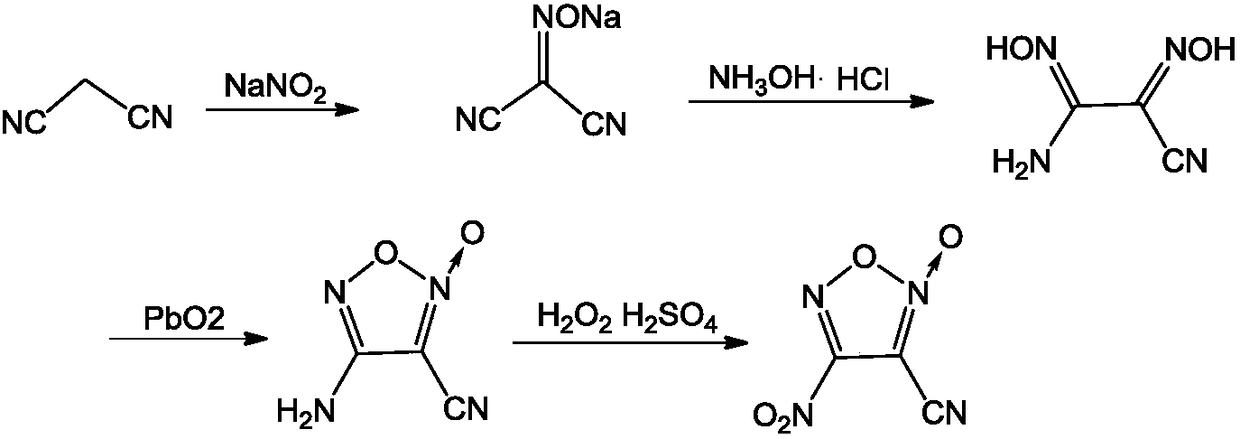
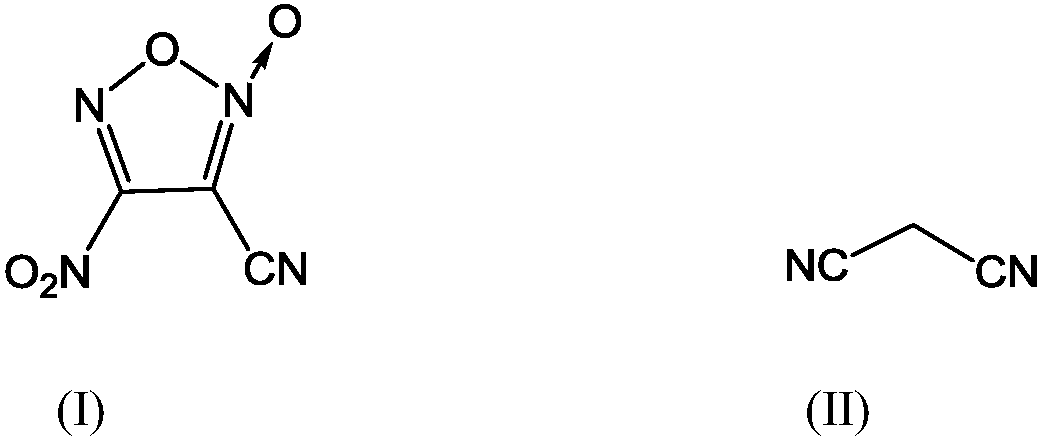A kind of synthetic method of 3-cyano-4-nitrofuroxan
A technology of nitrofuran and aminofuran oxide, applied in the direction of organic chemistry, can solve the problems of low reaction yield, cumbersome post-treatment, long reaction steps, etc., and achieve the effect of simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Under stirring, add 30g (454mmol) malononitrile and 37g (536mmol) sodium nitrite into 50mL water at a temperature of 0°C, then add 4mL (70mmol) of glacial acetic acid dropwise, and heat up to 20°C after the addition After reacting for 4 hours, the reaction liquid was extracted with ethyl acetate (100 mL×4) and dried. After filtration, the filtrate was distilled under reduced pressure to obtain 47.0 g of malononitrile oxime sodium salt, with a yield of 88.6% and a purity of 98.1%;
[0026] Structure Identification:
[0027] Infrared Spectrum: IR(KBr,cm -1 )ν: 2229, 1633, 1347;
[0028] NMR spectrum: 13 CNMR (CDCl 3 ,125MHz), δ:115.76,110.59,109.54;
[0029] Elemental Analysis: Structural Formula C 3 N 3 ONa
[0030] Theoretical value: C 30.79, N 35.90
[0031] Measured value: C 30.56, N 35.79;
[0032] The above-mentioned structural identification data confirm that the material obtained in this step is indeed malononitrile oxime sodium salt;
[0033] (2) Un...
Embodiment 2
[0062] (1) Under stirring, add 30g (454mmol) malononitrile and 35g (550mmol) sodium nitrite into 50mL water at a temperature of 0°C, then add 5mL (87mmol) of glacial acetic acid dropwise, and heat up to 20°C after the addition After reacting for 5 hours, the reaction liquid was extracted with ethyl acetate (100 mL×4) and dried. After filtration, the filtrate was distilled under reduced pressure to obtain 43.0 g of malononitrile oxime sodium salt, with a yield of 80.1% and a purity of 99.2%;
[0063] (2) Under stirring, add 13g (131.5mmol) of malononitrile oxime sodium salt to 250mL of absolute ethanol, then add 8.70g (125mmol) of hydroxylamine hydrochloride, heat up to reflux reaction for 4h after the addition, remove the solid by filtration, reduce Pressure distillation yielded 12.1 g of white solid 1-amino-2-cyanodioxime, yield 71.8%, purity 98.5%, m.p.: 99°C-100°C;
[0064] (3) Under stirring, dissolve 5.3 g (42 mmol) of 1-amino-2-cyanodioxime in 125 mL of ether, slowly add...
Embodiment 3
[0068] (1) Under stirring, add 30g (454mmol) malononitrile and 38g (507mmol) sodium nitrite into 50mL water at a temperature of 0°C, then add 3mL (52mmol) of glacial acetic acid dropwise, and heat up to 20°C after the addition After reacting for 4 hours, the reaction solution was extracted with ethyl acetate (100 mL×4) and dried. After filtration, the filtrate was distilled under reduced pressure to obtain 32.0 g of malononitrile oxime sodium salt, with a yield of 59.9% and a purity of 98.6%;
[0069] (2) Under stirring, add 13g (131.5mmol) of malononitrile oxime sodium salt to 320mL of absolute ethanol, then add 9.39g (135mmol) of hydroxylamine hydrochloride, heat up to reflux reaction for 5h after the addition, remove the solid by filtration, reduce Pressure distillation yielded 13.2 g of white solid 1-amino-2-cyanodioxime, yield 78.5%, purity 98.4%, m.p.: 99°C-100°C;
[0070] (3) Under stirring, 1-amino-2-cyanodioxime 5.3g (42mmol) was dissolved in 140mL ether, slowly added...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com