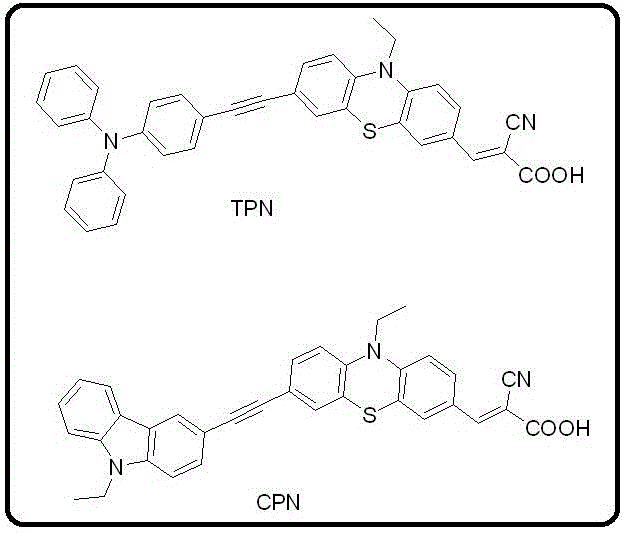
Double-donor aromatic amine photosensitizer and application thereof in LED visible light curing
An aromatic amine, electron donating technology, applied in the field of photofunctional organic molecules, can solve the problems of poor sensitivity improvement effect, long photocuring time, low photocuring efficiency, etc. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
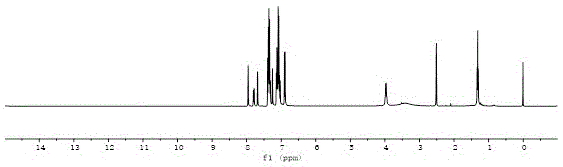
[0040] Synthesis of Sensitizer TPN
[0041] The synthesis process is shown in the following formula:
[0042]
[0043] Add N-ethyl-6-bromophenothiazine-3-aldehyde (0.2409g, 0.8mmol), Pd(PPh 3 ) 2 Cl 2 (8.5mg, 0.012mmol), CuI (7.6mg, 0.04mmol), triphenylphosphine (10.5mg, 0.04mmol), TEA10ml and DMF40ml, under the protection of nitrogen, dissolve triphenylamine acetylene (0.2574g, 0.96mmol) Add DMF20ml dropwise to the system, 85C 。Heat the oil bath until the reaction of N-ethyl-6-bromophenothiazine-3-aldehyde is complete. After the reaction system is cooled, it is poured into water, filtered, and dried in the air. Purified by column chromatography (PE:DCM=4:1) to obtain an orange-yellow solid. Under nitrogen protection, orange solid (0.2611g, 0.5mmol), cyanoacetic acid (0.2102g, 2.5mmol), 0.5ml of piperidine and 20ml of chloroform were sequentially added to a 100ml one-necked bottle. Reflux until the reaction of the raw material is complete, configure hydrochloric acid ...
Embodiment 2
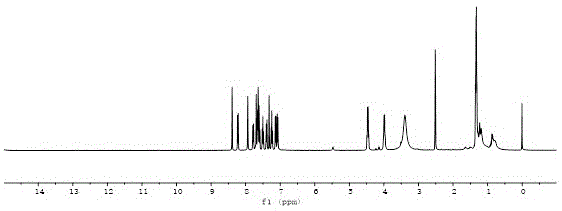
[0046] Synthesis of Sensitizer CPN
[0047] The synthesis process is shown in the following formula:
[0048]
[0049] Add N-ethyl-6-bromophenothiazine-3-aldehyde (0.2409g, 0.8mmol), Pd(PPh 3 ) 2 Cl 2 (8.5mg, 0.012mmol), CuI (7.6mg, 0.04mmol), triphenylphosphine (10.5mg, 0.04mmol), TEA10ml and DMF40ml, under the protection of nitrogen, dissolve N-ethylcarbazolyne (0.2103g, 0.96mmol) DMF20ml dropwise into the system, 85C 。 Heat the oil bath until the reaction of N-ethyl-6-bromophenothiazine-3-aldehyde is complete. After the reaction system is cooled, it is poured into water, filtered, and dried in the air. Purified by column chromatography (PE:DCM=4:1) to obtain an orange-yellow solid. Under the protection of nitrogen, orange solid (0.2361g, 0.5mmol), cyanoacetic acid (0.2102g, 2.5mmol), 0.5ml of piperidine and 20ml of chloroform were sequentially added to a 100ml one-necked bottle. Reflux until the reaction of the raw material is complete, prepare the hydrochloric aci...
Embodiment 3
[0052] The proportion of each component of a visible light initiating system that initiates free radical curing:
[0053] Photosensitizer TPN: 0.2wt%;
[0054] Free radical photoinitiator: 2wt%;
[0055] Additives: 0.2wt%.
[0056] Prepare the visible light triggering system according to the above-mentioned ratio, and add the visible light triggering system to the free radical polymerizing monomer: TPGDA based on the weight of the free radical polymerizing monomer as 100%, and mix thoroughly to obtain a transparent and clear photocuring reaction solution. Add the prepared light-curing system into a rubber ring mold with a thickness of 1.8mm and a diameter of 1.5mm, fix it with two clean glass pieces, and irradiate it with 455nm and 532nm laser diodes respectively to ensure that the sample and the excitation light source are consistent. The distance is 15cm. In order to ensure the credibility of the experimental results, three NIR tests were performed on each photocuring sys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com