Single-component flavonol sulfonate visible light initiator as well as preparation method and application thereof
A technology of flavonol sulfonate and initiator, which is applied in the direction of organic chemistry, can solve the problems of incomplete curing, poor solubility, low photocuring efficiency, etc., to reduce the cost of photocuring, reduce cytotoxicity, and improve the molecular structure simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
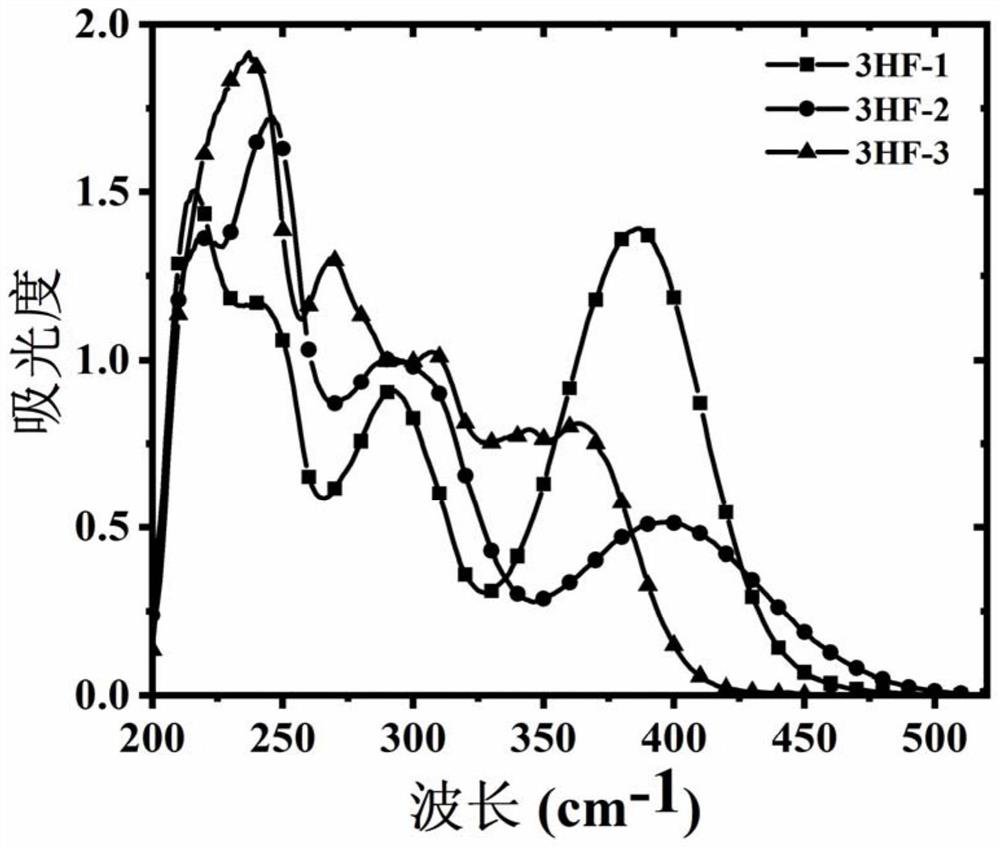
Image
Examples
Embodiment 1
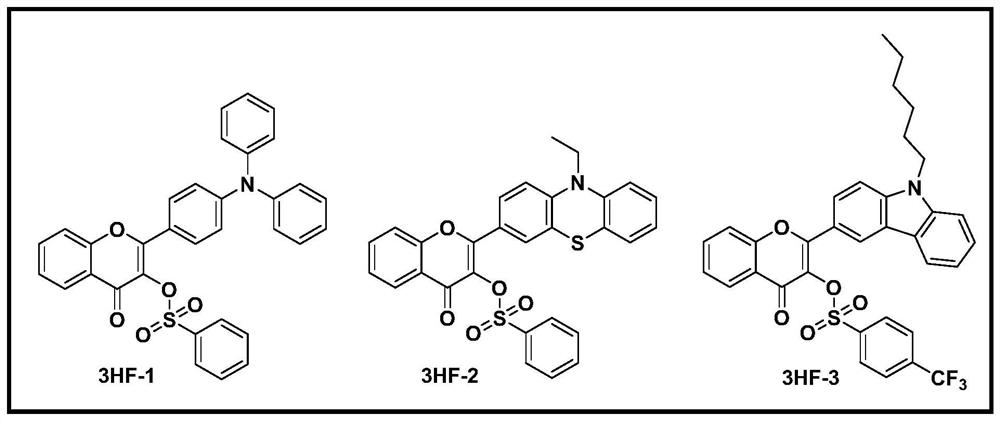
[0050] Synthesis of Initiator 2-(4-(Diphenylamino)phenyl)-4-oxo-4H-benzofuran-3-ylbenzenesulfonate
[0051] The synthesis process is shown in the following formula:
[0052]
[0053]38.6 mL of DMF, 37.3 mL of phosphorus oxychloride, and 24.8 g of triphenylamine were added to a 500-ml single-neck bottle, dissolved in 1,2-dichloroethane, and heated to 80°C. TLC detected the disappearance of the starting material, poured the reaction solution into 100 mL of ice water, adjusted the pH value to neutrality with sodium hydroxide, extracted with dichloromethane several times, and concentrated to obtain 4-(diphenylamino)benzaldehyde.
[0054] In a 500ml round-bottomed flask, add 12g of sodium hydroxide, dissolve in 30ml of water, reduce the temperature to room temperature, then add 20ml of ethanol, weigh 4.86g of 2-hydroxyacetophenone and 9.78g of 4-(diphenylamino)benzaldehyde and mix, Dissolve and dilute with 70 ml of ethanol, add half of the mixed solution dropwise to the round-b...
Embodiment 2
[0060] Synthesis of Initiator 2-(10-ethyl-10H-phenothiazin-3-yl)-4-oxo-4H-chromium-3-ylbenzenesulfonate
[0061] The synthesis process is shown in the following formula:
[0062]
[0063] 38.6 mL of DMF, 37.3 mL of phosphorus oxychloride, and 23.0 g of 10-ethyl-10H-phenothiazine were added to a 500-ml single-neck bottle, dissolved in 1,2-dichloroethane, and heated to 80°C. TLC detected the disappearance of the starting material, poured the reaction solution into 100 mL of ice water, adjusted the pH value to neutrality with sodium hydroxide, extracted several times with dichloromethane, and concentrated to obtain 10-ethyl-10H-phenothiazine-3- formaldehyde.
[0064] Add 12g of sodium hydroxide to a 500ml round-bottomed flask, dissolve in 30ml of water, reduce the temperature to room temperature, then add 20ml of ethanol, weigh 4.86g of 2-hydroxyacetophenone and 9.13g of 10-ethyl-10H-phenothiazine- 3-formaldehyde was mixed, dissolved and diluted with 70 ml of ethanol, half o...
Embodiment 3
[0070] Synthesis of Initiator 2-(9-hexyl-9H-carbazol-3-yl)-4-oxo-4H-benzofuran-3-yl 4-(trifluoromethyl)benzenesulfonate
[0071] The synthesis process is shown in the following formula:
[0072]
[0073] 38.6 mL of DMF, 37.3 mL of phosphorus oxychloride, and 25.4 g of 9-hexyl-9H-carbazole were added to a 500-ml single-neck bottle, dissolved in 1,2-dichloroethane, and heated to 80°C. TLC detected the disappearance of the raw material point, poured the reaction solution into 100 mL of ice water, adjusted the pH value to neutrality with sodium hydroxide, extracted several times with dichloromethane, and concentrated to obtain 9-hexyl-9H-carbazole-3-carbaldehyde.
[0074] Add 12g of sodium hydroxide to a 500ml round-bottomed flask, dissolve in 30ml of water, reduce the temperature to room temperature, then add 20ml of ethanol, weigh 4.86g of 2-hydroxyacetophenone and 9.99g of 9-hexyl-9H-carbazole-3- Formaldehyde was mixed, dissolved and diluted with 70 ml of ethanol, half of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com