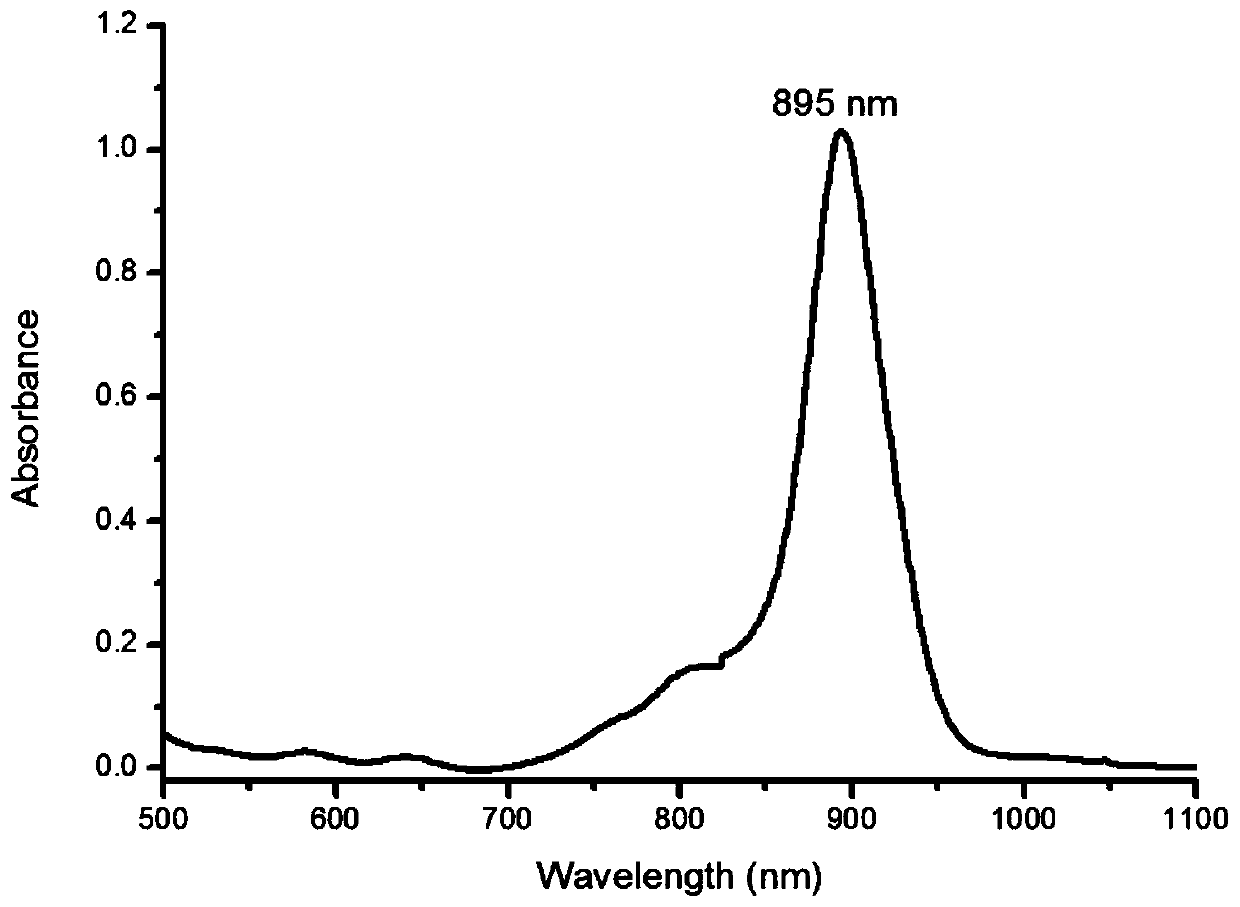
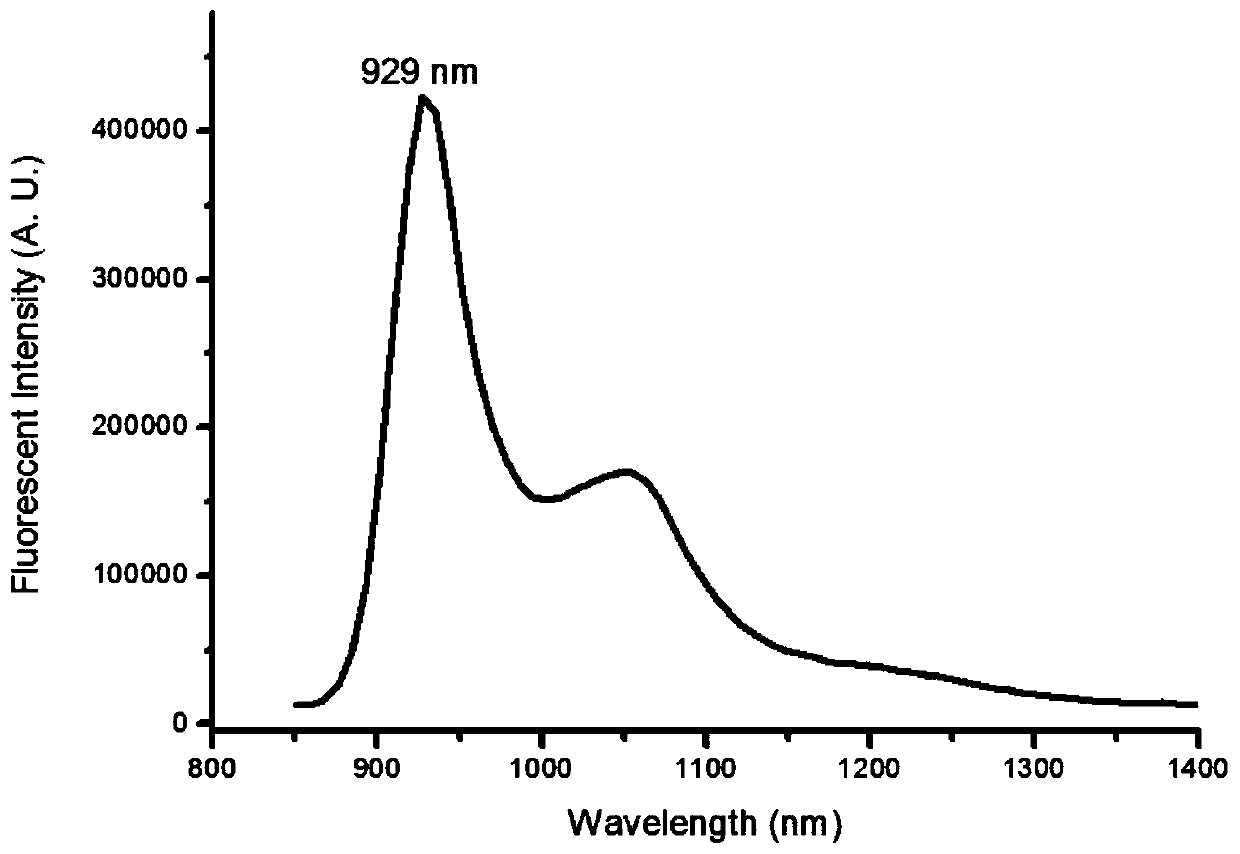
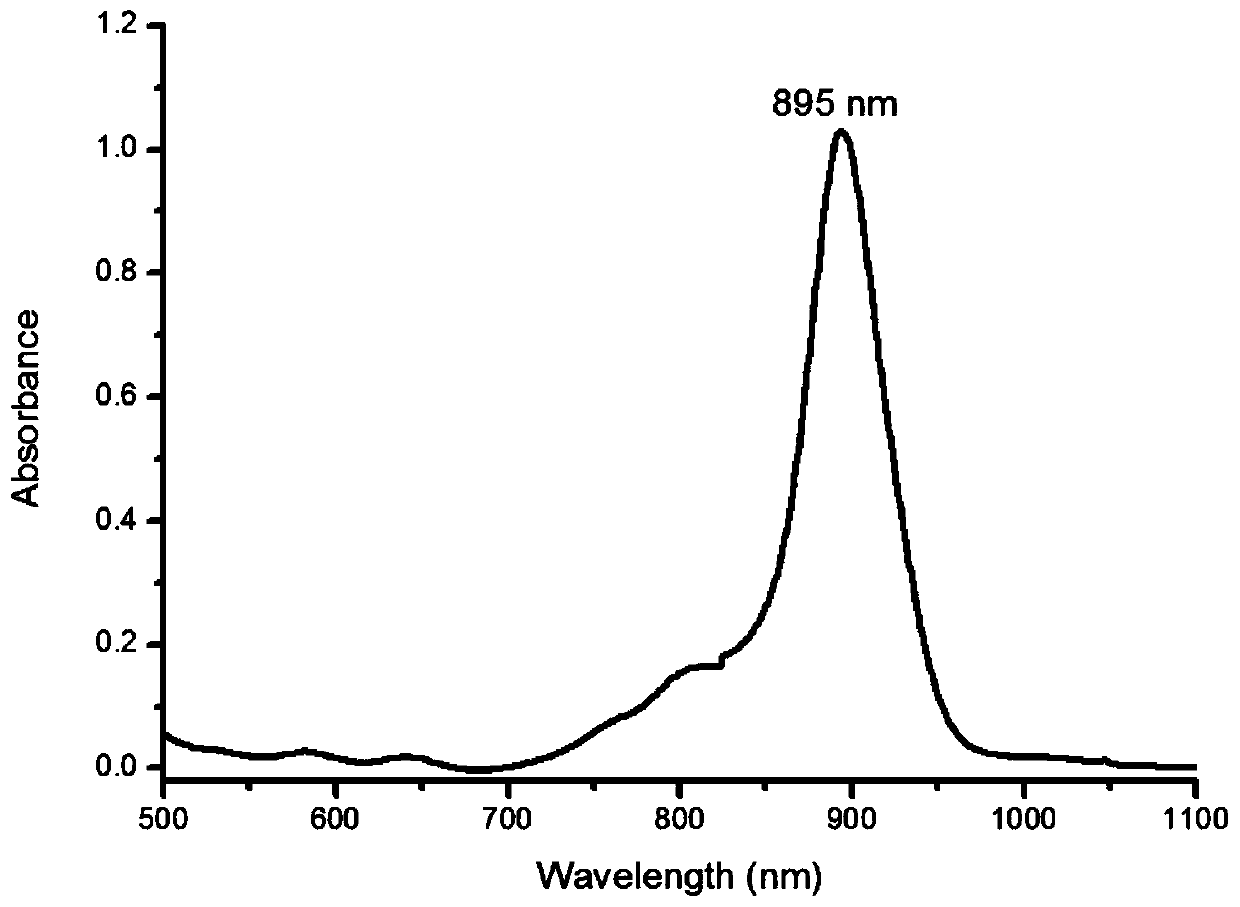
Near-infrared two-zone squaric acid quinoline dye and preparation method thereof
A squaraine quinoline, near-infrared technology, applied in organic dyes, chemical instruments and methods, methine/polymethine dyes, etc., can solve the problems of poor stability, low quantum yield, complex synthesis process, etc. Low effect, simple synthesis method and high molar extinction coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Weigh 0.5 g of 6-bromo-4-methylquinoline into a 50 ml flask, and add 20 ml of dry acetonitrile. After dissolving, 0.308 g of bromobutane was added, and the mixture was heated and refluxed at 70° C. to react for 6 hours. After the reaction was completed, most of the acetonitrile was removed by distillation under reduced pressure, the product was dropped into anhydrous ether, and a yellow solid was precipitated. After suction filtration, the filter cake was washed with anhydrous ether for 3 times to obtain a pure quinoline compound with a yield of 95%.
[0031] 1 H NMR (400MHz, CDCl 3 )δ(ppm)9.66(1H,d),8.68(1H,d),8.28(1H,d),8.15(1H,d),7.85(1H,d),5.56(2H,t),4.15(2H ,m),3.32(3H,s),1.79(2H,m),1.36(3H,t).ESI-MScalculated for C 14 H 17 BrN:278.05found:301.02[M+Na] + .
[0032] (2) 1 gram of malononitrile was weighed and dissolved in 50 ml of benzene. After being dissolved, 2.83 grams of diethyl squaraine was slowly added dropwise, and 1.67 grams of triethylamine was...
Embodiment 2
[0038](1) Weigh 0.5 g of 6-bromo-4-methylquinoline into a 50 ml flask, and add 20 ml of dry acetonitrile. After being dissolved, 0.414 g of iodobutane was added, and the mixture was heated and refluxed at 70° C. to react for 6 hours. After the reaction was completed, most of the acetonitrile was removed by distillation under reduced pressure, the product was dropped into anhydrous ether, and a yellow solid was precipitated. After suction filtration, the filter cake was washed with anhydrous ether for 3 times to obtain a pure product quinoline compound with a yield of 90%.
[0039] (2) 1 gram of malononitrile was weighed and dissolved in 50 ml of benzene, 2.83 grams of diethyl squaraine was slowly added dropwise after being dissolved, and 2.9 grams of triethylamine was added after the dropwise addition was completed. The solution was reacted at room temperature for 12 hours. After the reaction was completed, the solution was evaporated to dryness, and 10 ml of benzene was add...
Embodiment 3
[0042] (1) Weigh 0.5 g of 6-bromo-4-methylquinoline into a 50 ml flask, and add 20 ml of dry acetonitrile. After dissolving, 0.5 g of bromooctane was added, and the mixture was heated and refluxed at 70° C. to react for 6 hours. After the reaction was completed, most of the acetonitrile was removed by distillation under reduced pressure, the product was dropped into anhydrous ether, and a yellow solid was precipitated. After suction filtration, the filter cake was washed with anhydrous ether for 3 times to obtain a pure quinoline compound with a yield of 92%.
[0043] (2) 1 gram of malononitrile was weighed and dissolved in 50 ml of benzene, 2.83 grams of diethyl squaraine was slowly added dropwise after being dissolved, and 2.9 grams of triethylamine was added after the dropwise addition was completed. The solution was reacted at room temperature for 12 hours. After the reaction was completed, the solution was evaporated to dryness, and 10 ml of benzene was added for recrys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com