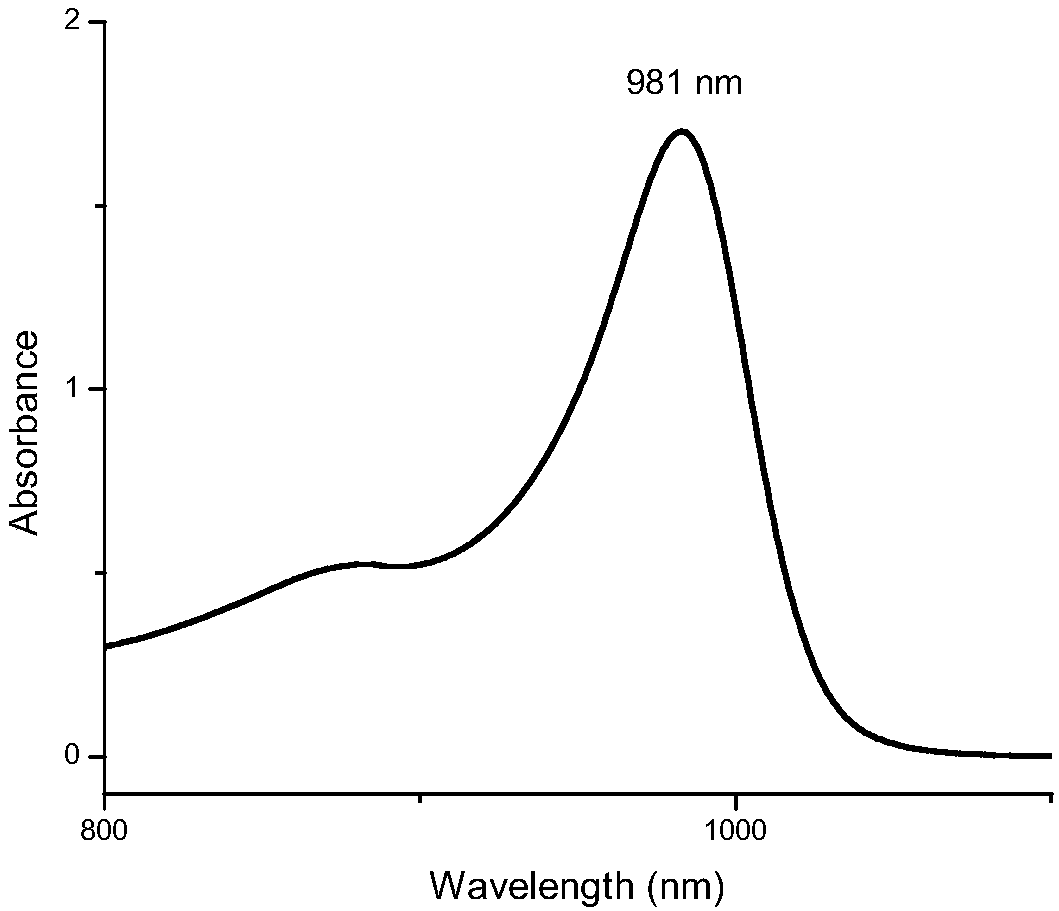
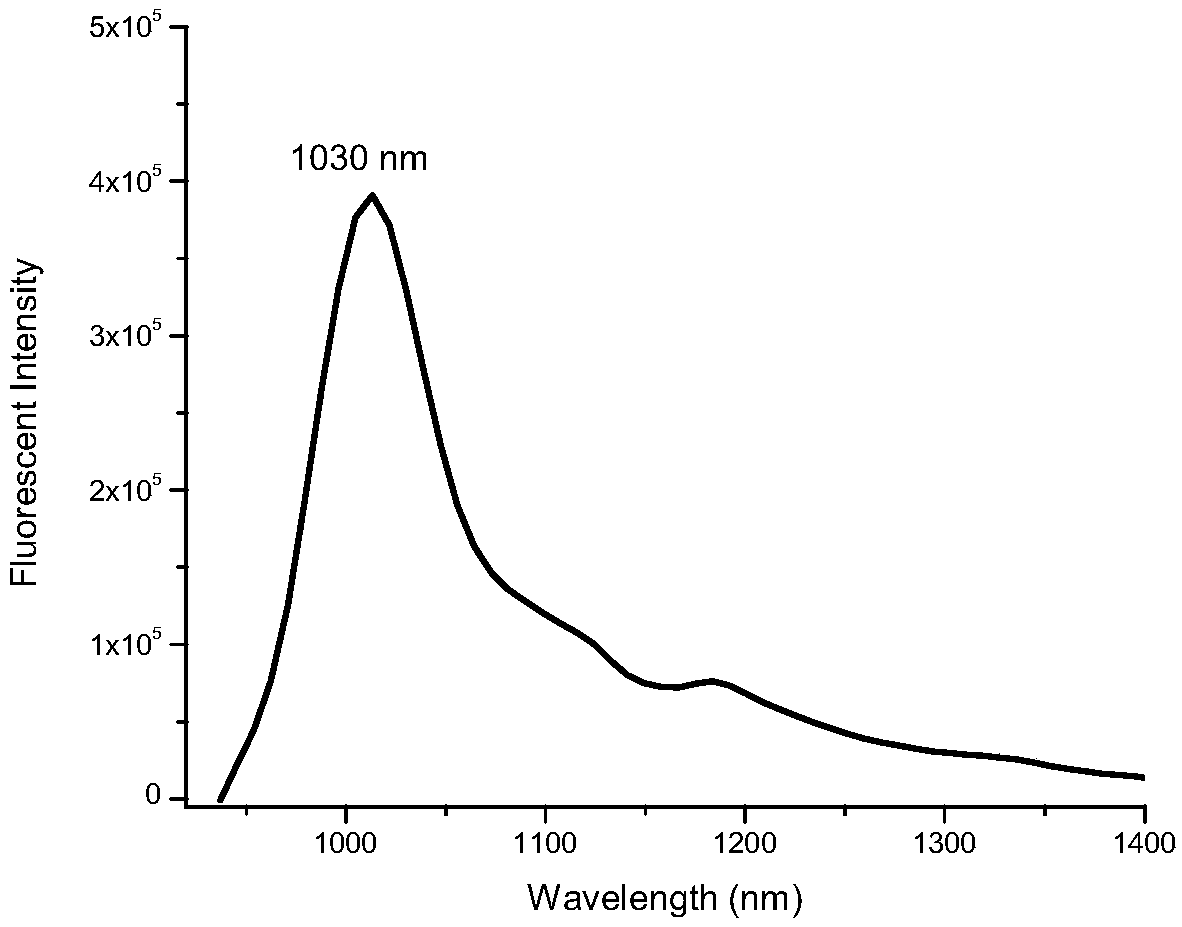
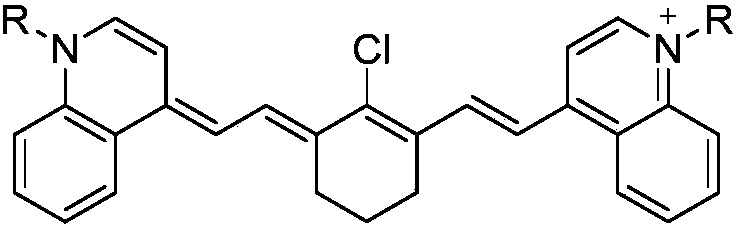
Preparation method of quinoline heptamethine cyanine dye
A technology of quinoline heptamethine and dyes, which is applied in the field of preparation of quinoline heptamethine dyes, can solve the problems of short maximum absorption wavelength and fluorescence emission wavelength, low sensitivity, etc., achieve good photostability, simple synthesis method, The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Weigh 3 g of 4-methylquinoline in a 100 ml flask, add 50 ml of dry acetonitrile. After dissolving, 2.26 g of ethyl bromide was added, and the mixture was reacted under reflux for 6 hours. After the reaction was completed, most of the acetonitrile was removed under vacuum distillation, and the product was dropped into anhydrous ether, and a yellow solid was precipitated. After suction filtration, the filter cake was washed three times with anhydrous ether to obtain a pure product with a yield of 95%.
[0036] (2) Weigh 0.8 grams of the product in step (1), N-[(3-(anilinomethylene)-2-chloro-1-cyclohexen-1-yl)methylene]aniline hydrochloride 0.5 grams, 0.9 grams of sodium acetate, dissolved in 50 ml of ethanol. After solvent removal, the mixture was reacted under reflux for 12 hours. After the reaction was complete, the solvent was evaporated, and the product was purified by column chromatography (dichloromethane:methanol=95:5), dissolved again in dichloromethane, an...
Embodiment 2
[0040] (1) Weigh 3 g of 4-methylquinoline in a 100 ml flask, add 50 ml of dry acetonitrile. After dissolving, 3.9 g of ethyl iodide was added, and the mixture was reacted under reflux for 6 hours. After the reaction was completed, most of the acetonitrile was removed under vacuum distillation, and the product was dropped into anhydrous ether, and a yellow solid was precipitated. After suction filtration, the filter cake was washed three times with anhydrous ether to obtain a pure product with a yield of 96%.
[0041] (2) Weigh 0.8 grams of the product in step (1), N-[(3-(anilinomethylene)-2-chloro-1-cyclohexen-1-yl)methylene]aniline hydrochloride 0.5 grams, 0.9 grams of sodium acetate, dissolved in 50 ml of ethanol. After solvent removal, the mixture was reacted under reflux for 12 hours. After the reaction was complete, the solvent was evaporated, and the product was purified by column chromatography (dichloromethane:methanol=95:5), dissolved again in dichloromethane, and ...
Embodiment 3
[0043] (1) Weigh 3 g of 4-methylquinoline in a 100 ml flask, add 50 ml of dry acetonitrile. After dissolving, 5.24 g of dodecane bromide was added, and the mixture was reacted under reflux for 6 hours. After the reaction was completed, most of the acetonitrile was removed under vacuum distillation, and the product was dropped into anhydrous ether, and a yellow solid was precipitated. After suction filtration, the filter cake was washed three times with anhydrous ether to obtain a pure product with a yield of 95%.
[0044] (2) Weigh 0.8 grams of the product in step (1), N-[(3-(anilinomethylene)-2-chloro-1-cyclohexen-1-yl)methylene]aniline hydrochloride 0.5 grams, 0.9 grams of sodium acetate, dissolved in 50 ml of ethanol. After solvent removal, the mixture was reacted under reflux for 12 hours. After the reaction was complete, the solvent was evaporated, and the product was purified by column chromatography (dichloromethane:methanol=95:5), dissolved again in dichloromethane,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com