Cysteine detection reagent and preparation method thereof
A detection reagent, cysteine technology, applied in the fields of chemical synthesis and biological analysis and detection, can solve the problems of complex preparation, difficult to meet commercial application requirements, and low sensitivity of detection reagents, and achieve the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
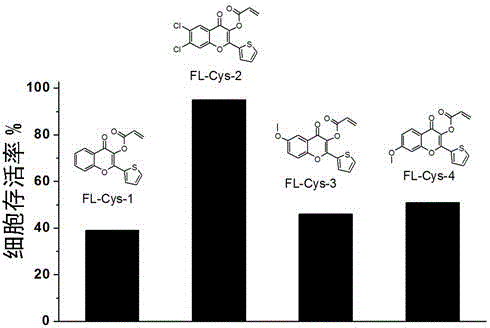
[0038] Synthesis of FL-Cys-1: Add 10 mmoles of hydroxyacetophenone and 10 mmoles of 2-formylthiophene to 20 ml of ethanol, then dropwise add 20 ml of 30% potassium hydroxide solution , stirred at room temperature for 10 hours after the dropwise addition; after stirring, the reaction solution was cooled, and 15 milliliters of hydrogen peroxide solution with a mass fraction of 30% was slowly added dropwise, stirred at room temperature for 24 hours, and neutralized with dilute hydrochloric acid with a concentration of 1 mol / liter. After the precipitate was filtered and dried, it was recrystallized in ethanol to obtain an intermediate product; then 5 mmoles of the intermediate was added to 10 ml of dry dichloromethane, 1 ml of triethylamine was added, and 3 ml of it was slowly added dropwise in an ice-water bath Acryloyl chloride, after reacting for half an hour, continue to react at room temperature for 4 hours, add 20 ml of saturated aqueous solution of sodium carbonate, extract ...
Embodiment 2
[0040] Synthesis of FL-Cys-2: Add 10 millimoles of 1-hydroxy-3,4-dichloroacetophenone and 10 millimoles of 2-formylthiophene to 20 milliliters of ethanol, then dropwise add 20 milliliters of 30% potassium hydroxide solution, after the dropwise addition, stir at room temperature for 10 hours; after stirring, cool the reaction solution, slowly add 15 ml of hydrogen peroxide solution with a mass fraction of 30%, stir at room temperature for 24 hours, and use a concentration of 1 mol / L neutralize the dilute hydrochloric acid, filter and dry the precipitate, and recrystallize in ethanol to obtain the intermediate product; then add 5 mmol of the intermediate to 10 ml of dry dichloromethane, add 2 ml of triethylamine, and Slowly add 2 ml of acryloyl chloride dropwise in the water bath, react for half an hour, continue to react at room temperature for 4 hours, add 20 ml of saturated aqueous solution of sodium carbonate, extract with dichloromethane, add anhydrous sodium sulfate to dry,...
Embodiment 3
[0042]Synthesis of FL-Cys-3: Add 10 millimoles of 1-hydroxy-4-hydroxymethylacetophenone and 10 millimoles of 2-formylthiophene to 20 milliliters of ethanol, then dropwise add 20 milliliters of 30% potassium hydroxide solution, stirred at room temperature for 10 hours after the dropwise addition; after stirring, the reaction solution was cooled, slowly added dropwise 15 ml of 30% hydrogen peroxide solution, stirred at room temperature for 24 hours, and diluted hydrochloric acid with a concentration of 1 mol / liter Neutralize, filter and dry the precipitate, recrystallize in ethanol to obtain the intermediate product; then add 5 mmoles of the intermediate to 10 ml of dry dichloromethane, add 1 ml of triethylamine, and slowly Add 2 ml of acryloyl chloride dropwise, react for half an hour, continue to react at room temperature for 12 hours, add 20 ml of saturated aqueous solution of sodium carbonate, extract with dichloromethane, add anhydrous sodium sulfate to dry, concentrate and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com