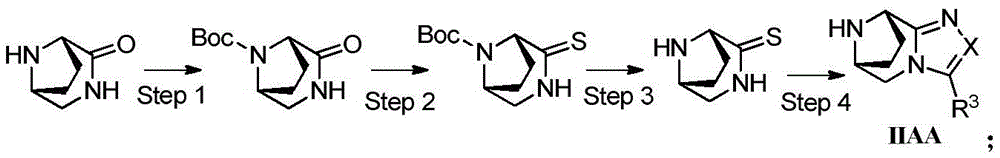
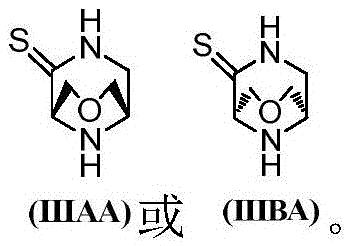
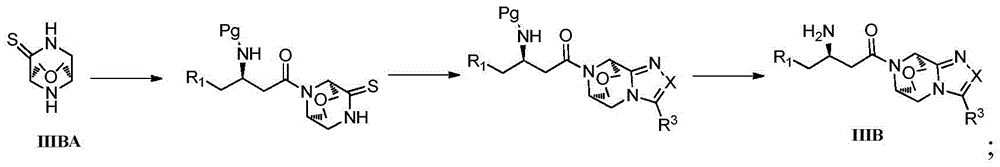
Intermediates of DPP-IV inhibitor
A next-step, isomer technology, applied in the field of intermediates of DPP-IV inhibitors, can solve problems such as inactivation and limitation of clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Example 1 (R)-3-amino-1-((6R,9S)-3-trifluoromethyl-6,7,8,9-tetrahydro-5H-6,9-cycloimine [1, 2,4]triazolo[4,3-a]azepine-10-alkyl)-4-(2,4,5-trifluorophenyl)-1-butanone trifluoroacetate ( Compound 1) Preparation
[0186]
[0187] Step 1: Methyl L-pyroglutamate (compound 1b)
[0188] Thionyl chloride (65 mL, 900 mmol) was added dropwise to 100 mL of methanol in an ice bath, followed by L-pyroglutamic acid 1a (58 g, 449 mmol). The reaction mixture was stirred at room temperature for 16 h, and then the solvent was dried under reduced pressure. Ethyl acetate (200 mL), sodium carbonate (50 g) and water (100 mL) were added and stirred for 1 hour. The organic phase was separated, and the aqueous phase was extracted with ethyl acetate (100 mL x 3). The organic phases were combined, dried over anhydrous sodium sulfate, and concentrated to obtain compound 1b, 51 g of a colorless liquid, with a yield of 79%.
[0189] MS (ESI) m / z: 144 (M+1); 1 HNMR (400mHz, CDCl 3 ): δ2.20-...
Embodiment 2
[0223] Example 2: (3R)-3-amino-1-(3-trifluoromethyl-6,7,9,10-tetrahydro-5H-6,10-cycloimine[1,2,4]tri Azolo[3,4-d][1,5]oxazocin-11-alkyl)-4-(2,4,5-trifluorophenyl)-1-butanone trifluoroacetate (compound 2)
[0224]
[0225] Step 1: (2S)-3-(3-tert-butoxycarbonylamino-2-hydroxypropoxy)-2-p-nitrobenzyloxycarbonylamino-propionic acid methyl ester (compound 2b)
[0226] To a solution of compound 2l (6.82 g, 35.7 mmol) and compound 2a (5 g, 17.8 mmol) in toluene (80 mL) was added boron trifluoride diethyl ether (0.3 mL, 48%) dropwise at room temperature. After stirring at room temperature for 2 h, the solvent was removed by concentration and column chromatography (silica gel, petroleum ether: ethyl acetate = 2:1) to obtain product 2b, 4.8 g of yellow oil, with a yield of 57%.
[0227] MS(ESI) m / z:472(M+1),416(M-56+1); 1 HNMR (400mHz, CDCl 3 ):δ8.22(d,J=8.4Hz,2H),7.52(d,J=8.4Hz,2H),5.08–5.13(m,1H),5.19-5.26(m,2H),4.42–4.58( m,1H),3.91–3.94(m,1H),3.78(s,3H),3.73-3.83(m,2H),3.54–...
Embodiment 3
[0257] Example 3 (R)-3-amino-1-((6R,9S)-3-methyl-6,7,8,9-tetrahydro-5H-6,9-cycloimine[1,2, 4] Triazolo[4,3-a]azepine-10-alkyl)-4-(2,4,5-trifluorophenyl)-1-butanone trifluoroacetate (compound 3 ) preparation
[0258]
[0259] Step 1: ((R)-4-carbonyl-4-((1S,5R)-2-thiocarbonyl-3,8-diazabicyclo[3.2.1]-8-octyl)-1-( tert-butyl 2,4,5-trifluorophenyl)-2-butyl)carbamate (Compound 3a)
[0260] Diisopropylethylamine ( 593mg, 4.6mmol), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (265mg, 1.38mmol), 1-hydroxybenzotriazole (186mg, 1.38mmol), Stir at room temperature for 1 hour. Then compound 1j (150 mg) was added to the reaction system and stirred overnight at room temperature. The reaction solution was washed with saturated brine, dried and concentrated. The crude product was purified by silica gel column (dichloromethane:methanol=80:1) to obtain compound 3a, white solid 170mg, yield 42%. MS (ESI) M / Z: 458 (M+H), 358 [(M+H)-Boc].
[0261] Step 2: ((R)-4-((6R,9S)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com