Preparation for inhibiting coronavirus infections
A coronavirus and preparation technology, applied in the direction of antiviral agents, medical preparations containing active ingredients, organic active ingredients, etc. The effect of suppressing infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, the preparation of plant extract compound formula virus infection inhibitor
[0039] Epigallocatechin gallate (EGCG), tannic acid and astragalus polysaccharide are uniformly mixed according to a mass ratio of 1:1:1.5 to obtain a plant extract compound formulation.
[0040] Before the experiment, it was dissolved in PBS buffer, made into 2560 μg / ml (the total concentration of EGCG, tannic acid and astragalus polysaccharide in the solution), filtered, and after sterilization, it was stored at -20°C for later use.
Embodiment 2
[0041] Embodiment 2, cytotoxicity test
[0042] In this example, the neutral red phagocytosis method was used to measure the cytotoxicity of the compound formulation prepared in Example 1 to mammalian cells. The specific operation is as follows:
[0043] The compound formulation preparation solution (2560 μg / ml) prepared in Example 1 was diluted step by step to obtain a total of 6 concentration. Then the dilutions of different dilutions were added to the 96-well cell culture plate in which 17Cl-1 cells were cultured and the cell density was about 80%, 100 μl in each well, and 4 duplicate holes were made for each dilution, and normal cells ( That is, no compound formula preparation) was used as a control. After 2 hours of action, the test solution was discarded, and the cell maintenance culture medium was added, and 200 μl was added to each well, and placed in a cell incubator for cultivation. After 48 hours, 0.1% (0.1g / 100 mL) 25 μl of neutral red, after reacting at 37° C....
Embodiment 3
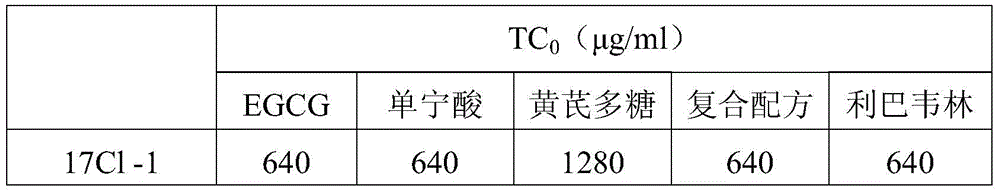
[0048] Embodiment 3, coronavirus infection inhibition test
[0049] In this embodiment, the CPE method is used to measure the inhibitory effect of the compound formulation prepared in Example 1 on coronavirus infection. The tested coronavirus is murine hepatitis coronavirus A59 strain.
[0050] Take a 96-well plate cultured with 17Cl-1 cells that have grown into a monolayer with a growth density of about 80%, pour off the culture medium, rinse the cells with PBS for 3 times, and use the three conditions of A, B, and C respectively. Add the compound formula preparation that embodiment 1 prepares:
[0051] A. Simultaneously with virus adsorption: put an equal volume of 2×100TCID 50 After mixing the virus liquid of the coronavirus with 2 times the concentration of the test substance solution, add 100 μl / well of the mixture to the cell culture plate, place it in the cell culture incubator, and discard it after the virus is adsorbed for 1 hour. After washing the cell surface 3 t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com