Method for synthesizing alpha, beta-unsaturated acid by using formic acid and alkine
An unsaturated and alkyne technology, applied in chemical instruments and methods, preparation of organic compounds, carboxylate preparation, etc., to achieve high efficiency, good industrial application prospects, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
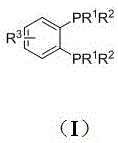
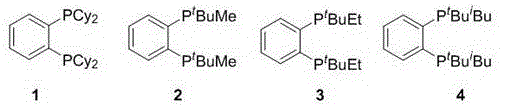
[0031] Preparation of Ligand 1
[0032]1,2-bis(dicyclohexyl)phosphinobenzene (1): Weigh 5 (280mg, 1mmol) in a dry and clean 50mL Schlenk bottle in the glove box. After taking it out, 10 mL of anhydrous THF was added, and the temperature was lowered to -78°C under the protection of nitrogen. Subsequently, cyclohexylmagnesium bromide (1MinTHF, 6mL) was added dropwise using a syringe. After the dropwise addition, the temperature was naturally raised to room temperature, and stirring was continued for 12 h. h 1 After the complete reaction was monitored by NMR, the workup was started. First drain the THF solution, then add n-hexane to dissolve it, and then add saturated ammonium chloride to quench the reaction. The organic phase was extracted with n-hexane. The organic phase was dried using anhydrous sodium sulfate. After removing the desiccant by suction filtration, the system was desolvated with a rotary evaporator to remove the oily liquid. Using petroleum ether and eth...
Embodiment 2
[0035] Preparation of Ligand 2 1,2-Di(tert-butylmethyl)phosphinobenzene (2): Weigh 5 (560mg, 2mmol) in a dry and clean 50mL Schlenk bottle in the glove box. After taking it out, 10 mL of anhydrous THF was added, and the temperature was lowered to -78°C under the protection of nitrogen. Subsequently, tert-butylmagnesium chloride (1.7MinEt 2 O, 2.4mL). After the dropwise addition, the temperature was naturally raised to room temperature, and stirring was continued for 5 h. h 1 After the complete reaction was monitored by NMR, methylmagnesium chloride (3MinTHF, 2.0mL) was added dropwise using a syringe, stirring was continued for 2h and post-treatment began. First drain the THF solution, then add n-hexane to dissolve it, and then add saturated ammonium chloride to quench the reaction. The organic phase was extracted with n-hexane. The organic phase was dried using anhydrous sodium sulfate. After removing the desiccant by suction filtration, the system was desolvated with ...
Embodiment 3
[0038] Preparation of Ligand 3 1,2-Di(tert-butylethyl)phosphinobenzene (3): Weigh 5 (560mg, 2mmol) in a dry and clean 50mL Schlenk bottle in the glove box. After taking it out, 10 mL of anhydrous THF was added, and the temperature was lowered to -78°C under the protection of nitrogen. Subsequently, tert-butylmagnesium chloride (1.7MinEt 2 O, 2.4mL). After the dropwise addition, the temperature was naturally raised to room temperature, and stirring was continued for 5 h. h 1 After the complete reaction was monitored by NMR, ethylmagnesium chloride (3MinTHF, 2.0mL) was added dropwise using a syringe. After the dropwise addition, the temperature was raised to reflux, and after stirring for 2h, post-treatment began. First drain the THF solution, then add n-hexane to dissolve it, and then add saturated ammonium chloride to quench the reaction. The organic phase was extracted with n-hexane. The organic phase was dried using anhydrous sodium sulfate. After removing the desicc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com