Method for preparing organosilicon by using channel reaction device
A channel reaction device and organosilicon technology, which is applied in the fields of organic chemistry, chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, etc. problems, to achieve the effect of increased yield, large output flexibility, and flexible production arrangements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
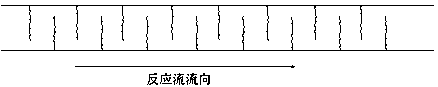
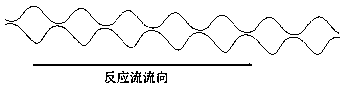
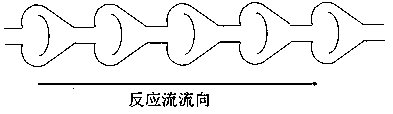
Image
Examples
Embodiment 1
[0060] Example 1 Preparation of 3-chloropropyl trichlorosilane
[0061] The raw materials are trichlorosilane and chloropropene, the main catalyst is chloroplatinic acid hexahydrate, the auxiliary agent is isopropanol and organic amine, and the molar ratio of trichlorosilane, chloropropene and chloroplatinic acid hexahydrate is 500000: 500000:1 , chloroplatinic acid hexahydrate is made into a solution of 1‰ with isopropanol and organic amine mixed solution with a volume ratio of 9:1; the channel reaction device is a baffle type, the activator is an organic amine, and the inner diameter of the channel is 6 mm, and the length is 60 meters, the flow rate of the reaction stream is 25Kg / h, and the reaction temperature is 115°C; fully mix the catalyst mixed solution with chloropropene, and pump chloropropene and trichlorosilane from the two inlets of the channel reaction device at the same time. Sampling and testing from the outlet of the channel reaction device showed that the cont...
Embodiment 2
[0062] Example 2 Preparation of 3-(2,3-epoxypropoxy)propyltrimethoxysilane
[0063] The raw materials are trimethoxysilane hydrogen and allyl glycidyl ether, the main catalyst is chloroplatinic acid hexahydrate, the auxiliary agents are tetrahydrofuran and isopropanol, trimethoxysilane hydrogen, allyl glycidyl ether and chloroplatinum hexahydrate The molar ratio of the acid is 400000:400000:1, and chloroplatinic acid hexahydrate is prepared into a 1‰ solution with a mixture of tetrahydrofuran and isopropanol at a volume ratio of 9:1; the channel reaction device is a baffle type, and the activator is an organic amine , the inner diameter of the channel is 6mm, the length is 60m, the flow rate of the reaction flow is 25Kg / h, and the reaction temperature is 90°C; the catalyst mixed solution is fully mixed with allyl glycidyl ether, trimethoxyhydrogen silicon and allyl Glycidyl ether is pumped in simultaneously from two inlets of the channel reaction device. Samples were taken fr...
Embodiment 3
[0064] Example 3 Preparation of 3-methacryloxypropyltrimethoxysilane
[0065] The raw materials are trimethoxysilane hydrogen and allyl methacrylate, the main catalyst is chloroplatinic acid hexahydrate, and the auxiliary agent is acetylacetone, trimethoxysilane hydrogen, allyl methacrylate and chloroplatinic acid hexahydrate. The ratio is 600000: 600000:1, chloroplatinic acid hexahydrate is prepared into a 1‰ solution with acetylacetone; the channel reaction device is peristaltic, the activator is 3-aminopropyltriethoxysilane, and the inner diameter of the channel is 8 mm , the length is 75 meters, the flow rate of the reaction flow is 32Kg / h, and the reaction temperature is 105°C; the catalyst mixture is fully mixed with allyl methacrylate, and trimethoxyhydrogen silicon and allyl methacrylate are simultaneously transferred from the channel The two inlets of the reaction device are pumped in. Samples were taken from the outlet of the channel reaction device for testing, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com