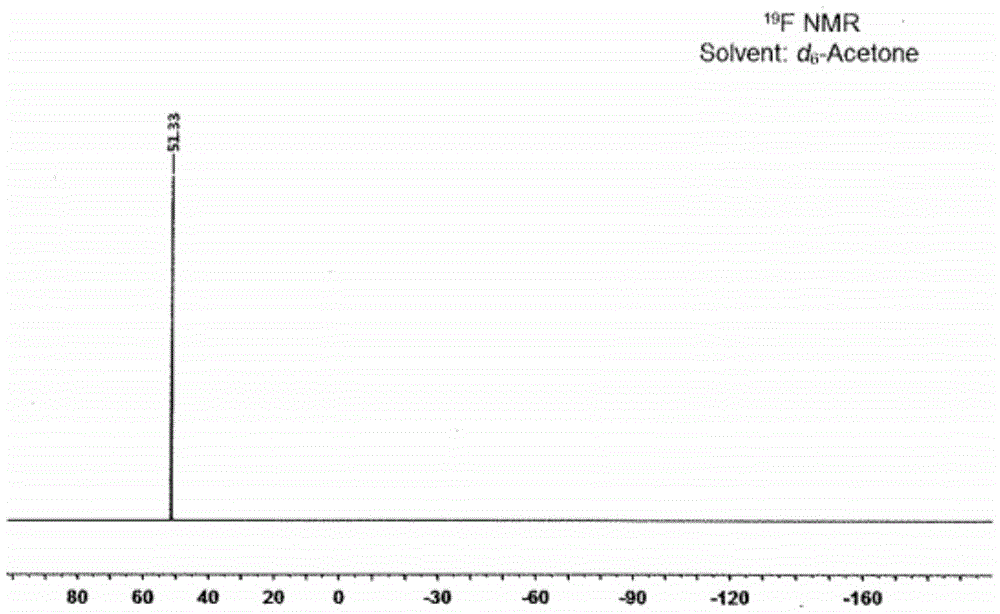
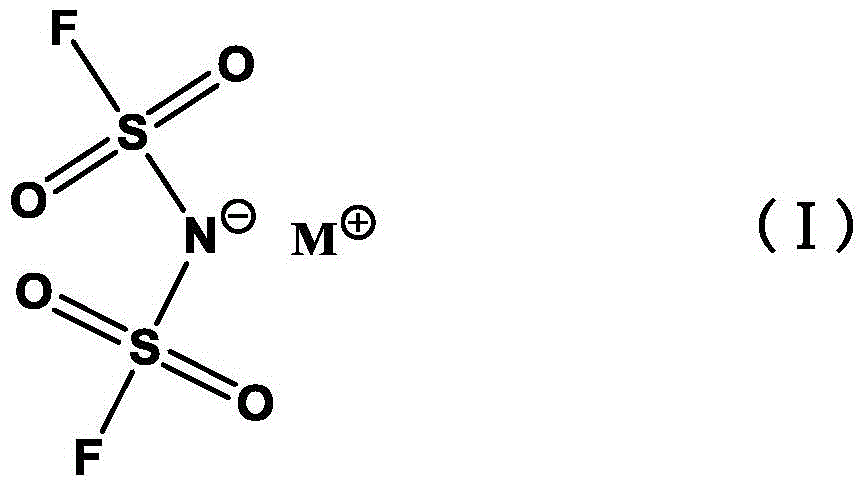
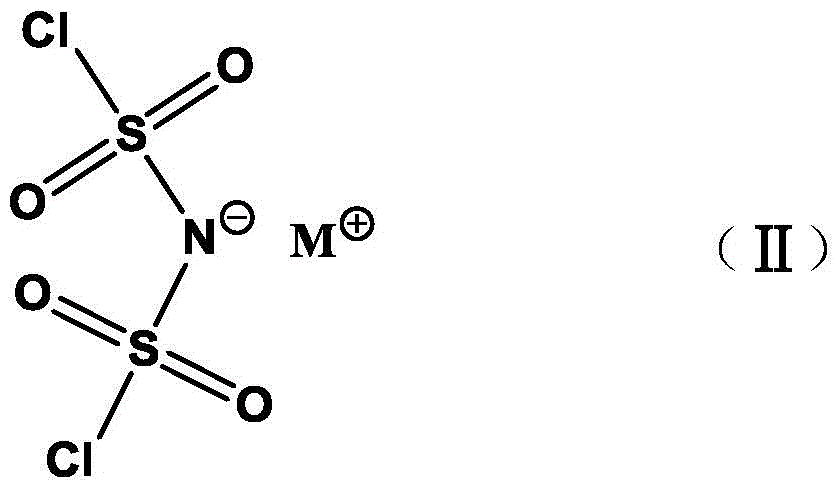
Preparation method of alkali metal salt of bis(fluorosulfonyl)amide
A technology of bisfluorosulfonimide and bischlorosulfonimide is applied in the field of preparation of alkali metal salts of bisfluorosulfonimide, and can solve the problems of difficult separation, difficult separation and purification, low fluorination efficiency and the like, To achieve the effect of easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Dichlorosulfonimide (HN(SO 2 Cl) 2 , the synthesis of HClSI)
[0034] The response is as follows:
[0035] ClSO 3 H+ClSO 2 NCO→HN(SO 2 Cl) 2 +CO 2 ↑
[0036] In a 500mL three-neck flask equipped with mechanical stirring, under nitrogen protection, add chlorosulfonic acid (163g, 1mol) and chlorosulfonyl isocyanate (155g, 1.1mol) in sequence, and heat the reaction at 100-110°C until carbon dioxide gas is produced . Then carry out vacuum distillation, collect the distillate at 112-114° C. / 2 mmHg, and obtain 190 g (90% yield) of bischlorosulfonimide (HClSI), which is colorless crystal at room temperature.
Embodiment 2
[0038] Synthesis of lithium bischlorosulfonyl imide (LiClSI)
[0039] The response is as follows:
[0040] HN(SO 2 Cl) 2 +LiCl→LiN(SO 2 Cl) 2 +HCl↑
[0041] According to the literature J.Inorg.Nucl.Chem.1977, 39,441 synthesis. In a 500mL three-necked flask equipped with mechanical stirring, under the protection of nitrogen flow, anhydrous 1,2-dichloroethane (200mL) and bischlorosulfonimide (107g, 0.5mol) were successively added, and then, at room temperature Under stirring, LiCl (43 g, 1 mol) was added in two portions, stirred at room temperature, at this time, HCl gas was released, and the reaction was continued at 60° C. for 10 hours until no HCl gas was generated. Then, the white solid was collected by filtration in a glove box, and the solid was washed 3 times with 1,2-dichloroethane (30 mL each). At 40° C., the oil pump was vacuum-dried for 10 hours to obtain 210 g of white powdery solid lithium bischlorosulfonimide (LiClSI) (yield 96%).
Embodiment 3-6
[0043] According to the above-mentioned steps of Example 2, replace LiCl with equimolar NaCl, KCl, RbCl and CsCl respectively, and other conditions remain unchanged, correspondingly obtain bischlorosulfonimide sodium (NaClSI), bischlorosulfonimide potassium (KClSI) , rubidium bischlorosulfonimide (RbClSI) and cesium bischlorosulfonimide (CsClSI). The results are shown in Table 1.
[0044]
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com