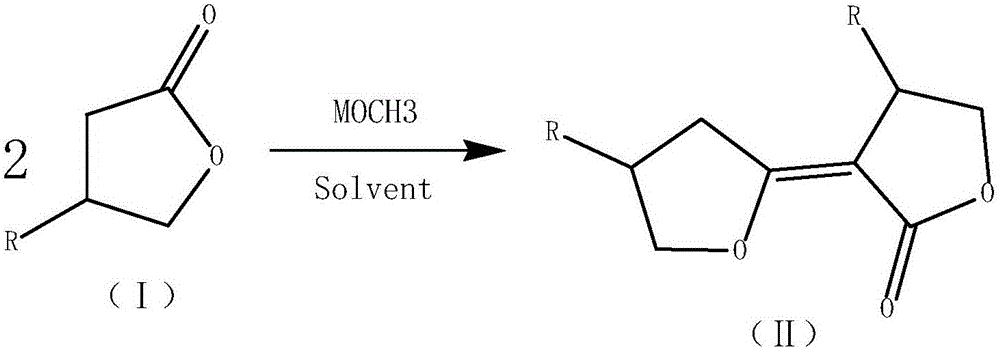
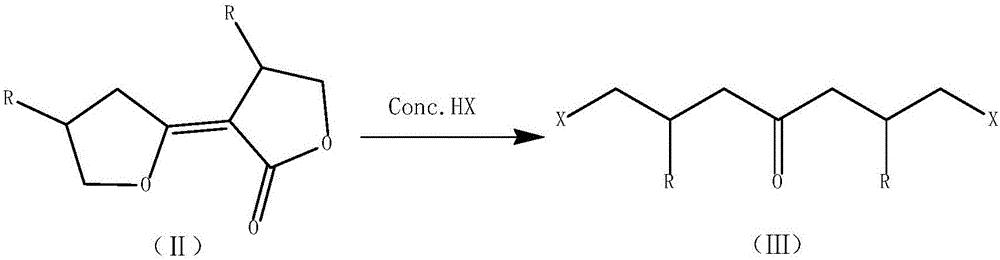
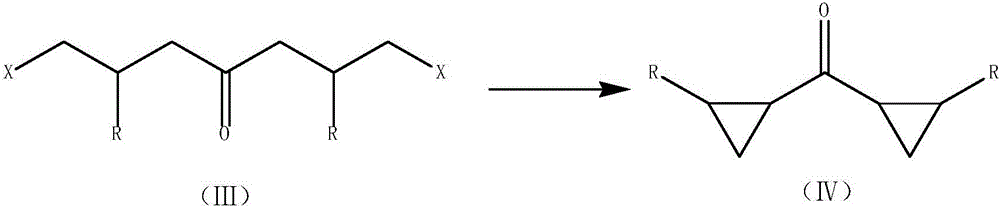
Modified synthetic method of dicyclopropyl ketone
A technology of dicyclopropyl ketone and synthesis method, which is applied in the field of improved preparation, can solve the problems of prone to material flushing accidents, unstable yield, poor controllability, etc., and achieve the reduction of water-soluble impurities, high product yield, and environmental protection The effect of pressure reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In a 1000mL four-necked bottle, equipped with a thermometer, an electric stirrer, a reflux condenser, and a U-shaped calcium chloride drying tube. 60 g of toluene and 48.8 g of solid sodium methoxide were added under stirring, and the internal temperature was raised to 75°C by heating, and 137.6 g of γ-butyrolactone was added dropwise. After the dropwise addition is completed, the temperature is raised to an internal temperature of 80-90°C and the reaction is carried out for more than 2 hours. Then cool down to room temperature, add 402g of pre-frozen concentrated hydrochloric acid, heat up to 85-90°C to react for more than 1 hour after adding, cool down, filter, and separate phases of the filtrate, and extract the water phase with 20g of toluene for washing the solid. The toluene phases were combined to obtain 182.4 g of the CrDCK / toluene solution in step (2), including 102.4 g of CrDCK and a yield of 70.0% (based on gamma-butyrolactone).
[0039] In a 1000mL four-nec...
Embodiment 2
[0041] In a 1000mL four-necked bottle, equipped with a thermometer, an electric stirrer, a reflux condenser, and a U-shaped calcium chloride drying tube. 80 g of toluene and 101.4 g of solid potassium tert-butoxide were added under stirring, and the internal temperature was raised to 75°C by heating, and 160.0 g of β-methyl-γ-butyrolactone was added dropwise. After the dropwise addition was completed, the temperature was raised to an internal temperature of 80-90°C and the reaction was carried out for 2 hours. Then cool down to room temperature, add pre-frozen concentrated hydrochloric acid 402g, heat up to an internal temperature of 85-90°C for 1 hour, cool down, filter, and separate phases of the filtrate. In the toluene phase, 319.3 g of the β-methyl-CrDCK / toluene solution of step (2) was obtained, including 219.3 g of CrDCK, and the yield was 65.0% (based on γ-butyrolactone).
[0042] Add 200g of water and 65g of solid sodium hydroxide to a 1000mL four-necked bottle, stir...
Embodiment 3
[0044]In a 1000mL four-necked bottle, equipped with a thermometer, an electric stirrer, a reflux condenser, and a U-shaped calcium chloride drying tube. While stirring, 90 g of toluene and 65.3 g of solid sodium ethoxide were added, heated to raise the internal temperature to 75°C, and 137.6 g of γ-butyrolactone was added dropwise. After the dropwise addition is completed, the temperature is raised to an internal temperature of 80-90°C and the reaction is carried out for more than 2 hours. Then cool down to room temperature, add 402g of pre-frozen concentrated hydrochloric acid, heat up to 85-90°C to react for more than 1 hour after adding, cool down, filter, and separate phases of the filtrate, and extract the water phase with 20g of toluene for washing the solid. The toluene phases were combined to obtain 209.5 g of the CrDCK / toluene solution in step (2), of which CrDCK was 99.5 g, and the yield was 68.0% (based on gamma-butyrolactone).
[0045] Add 180g of water and 64g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com