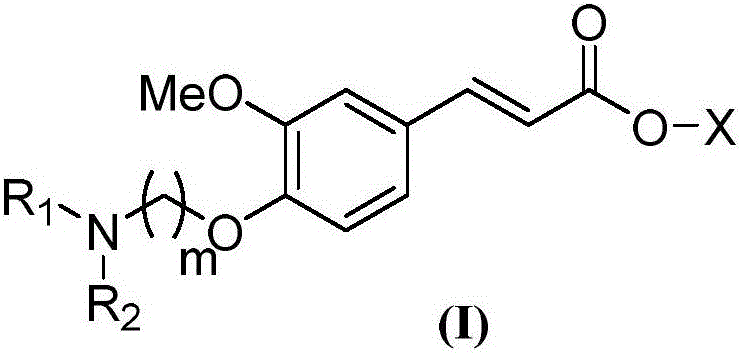
4-amine alkoxy-3-methoxyl cinnamic acid ester compound, preparation method and application of compound
A methoxycarnitine and amine alkoxy technology, which is applied in the field of drugs for the treatment and/or prevention of neurodegenerative-related diseases, can solve the problems of many toxic and side effects, a single target of action, and poor long-term efficacy in AD patients. Cell damage protection, significant effect of cell damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
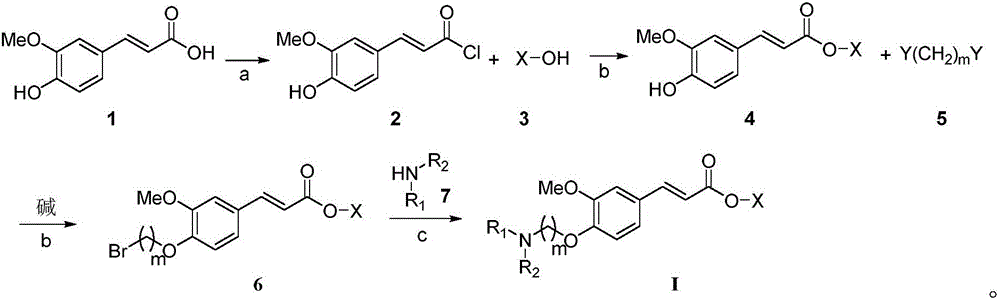
[0058] A preparation method of 4-aminoalkoxy-3-methoxycinnamate compound, comprising the following steps:
[0059] The first step, add ferulic acid, the first solvent, catalytic amount DMF and acylating agent in reaction bottle, the addition amount of catalyzer is 0.05% of ferulic acid weight, heats up and refluxes and stirs reaction, evaporates under reduced pressure and removes solvent, obtains Ferulic acid chloride;
[0060] In the second step, the above-mentioned ferulic acid chloride is dissolved in the second solvent, and the alkali and ethanol used in the first basic condition are added, and the reaction is stirred, and the reaction process is followed by TLC; after the reaction is completed, the solvent is evaporated under reduced pressure, and the residue is Add the second solvent, wash with saturated aqueous sodium carbonate and saturated aqueous sodium chloride successively, filter the organic layer after drying over anhydrous sodium sulfate, distill off the solvent...
Embodiment 15
[0065] Substantially the same as Example 7, the difference of the first step is: the catalyst used is the mixture of DMF, pyridine and aluminum chloride, the DMF add-on is 0.03% of the ferulic acid weight, the pyridine add-on is the ferulic acid 0.01% by weight, and the addition of aluminum trichloride is 0.005% by weight of ferulic acid. See Table 1-4 for the differences in other steps.
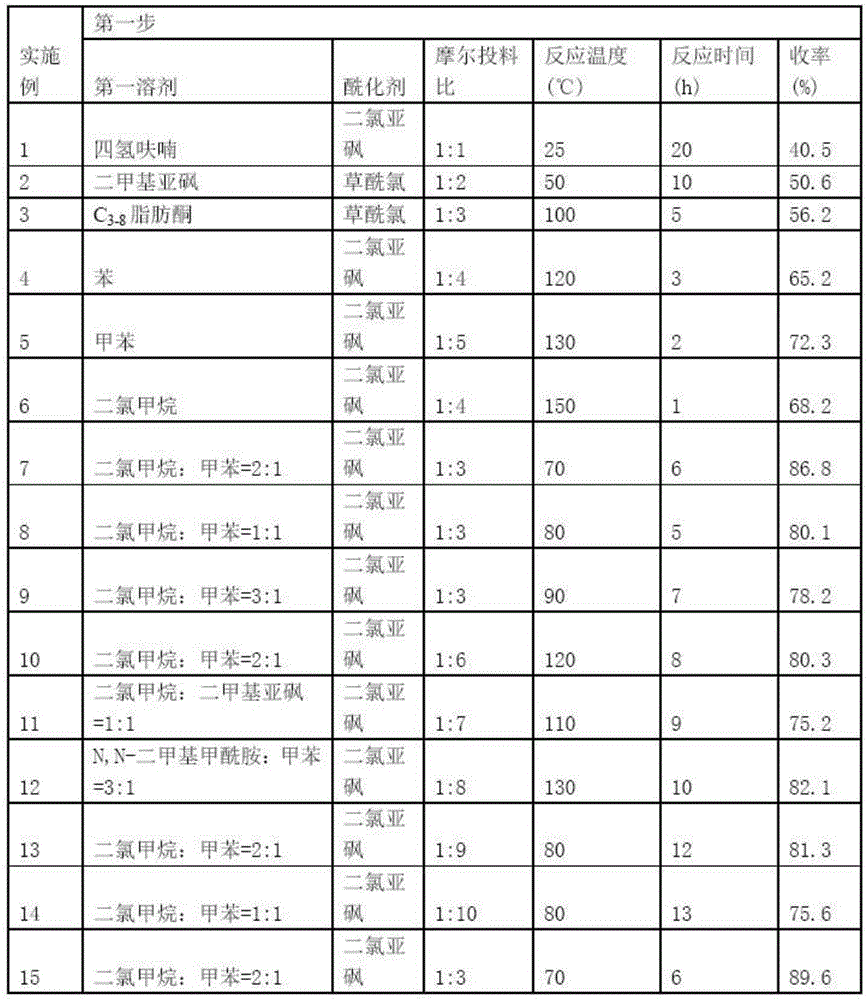
[0066] Table 1 Process conditions and results of the first step
[0067]
[0068] As can be seen from Table 1, the process conditions have a serious impact on the yield of the product, and selecting suitable process conditions has an important meaning to the product yield. It can be seen from Table 1 that the first solvent is dichloromethane:toluene with a volume ratio of 2:1, the acylating agent is thionyl chloride, the molar feeding ratio is 1:3, the reaction temperature is 70°C, and the reaction time is 6h. , the yield is 86.8%, and the yield is very high.
[0069] As can be seen fr...
Embodiment 16
[0080] The specific process conditions are the same as in Example 7, and the difference is the same as the investigation of different substituents. The specific substituents are shown in Table 5, and the obtained 4-aminoalkoxy-3-methoxycinnamate compounds have their chemical structures verified. 1 H-NMR, 13 Confirmed by C-NMR and ESI-MS.
[0081] Table 5 Different Substituent Experiments
[0082]
[0083]
[0084]
[0085]
[0086]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com