Rinsing liquid for nucleic acid extraction purification
A rinsing solution and nucleic acid technology, applied in the field of rinsing solution, can solve problems such as affecting nucleic acid stability and cross-contamination, and achieve the effects of reducing the chance of cross-contamination, eliminating the inhibitory effect, and reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
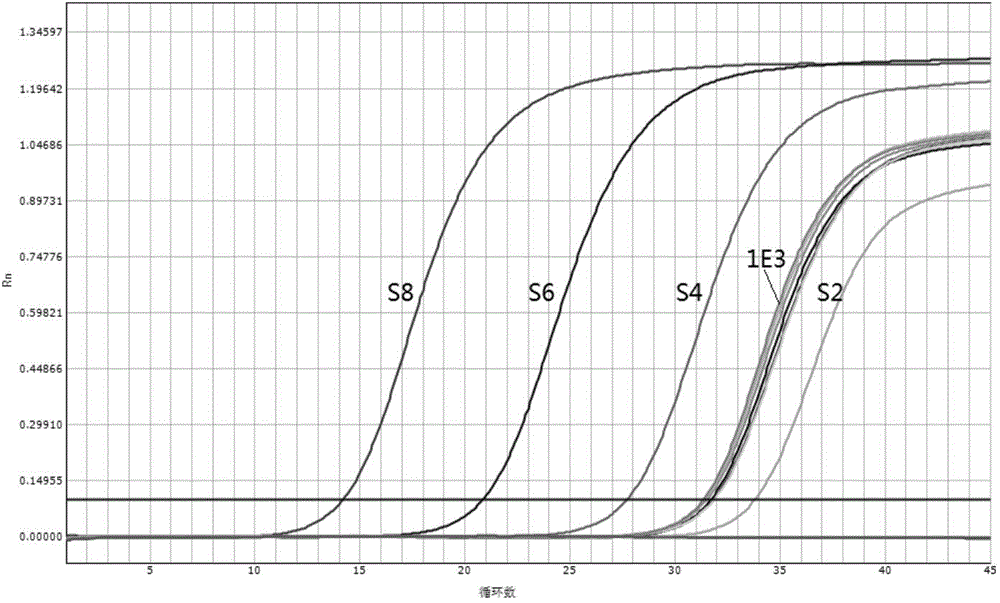
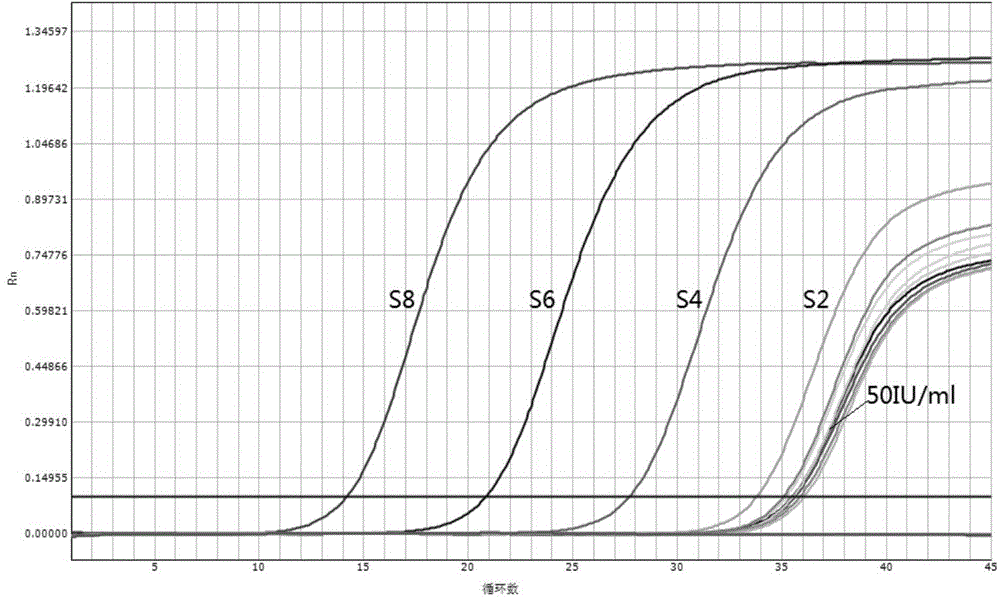
[0046] Embodiment 1. the application of rinse solution of the present invention in the quantitative detection of hepatitis B virus (HBV) nucleic acid
[0047] In this embodiment, the rinsing solution provided by the present invention is used to replace the rinsing solution in the commercially available HBV nucleic acid quantitative reagent, and the drying step of the rinsing solution is omitted in the nucleic acid extraction process, and the HBV international standard is quantitatively detected. From the results of the test Whether the rinsing solution provided by the present invention is judged whether it is feasible in terms of accuracy and sensitivity, the specific research materials are as follows:
[0048] (1) "Hepatitis B Virus Nucleic Acid Detection Kit (Fluorescent Quantitative PCR Method)" produced by Shanghai Haoyuan Biotechnology Co., Ltd. (Medical Device Registration Certificate Number: National Food and Drug Administration (Zhun) No. 2013 No. 3402077); This produc...
Embodiment 2
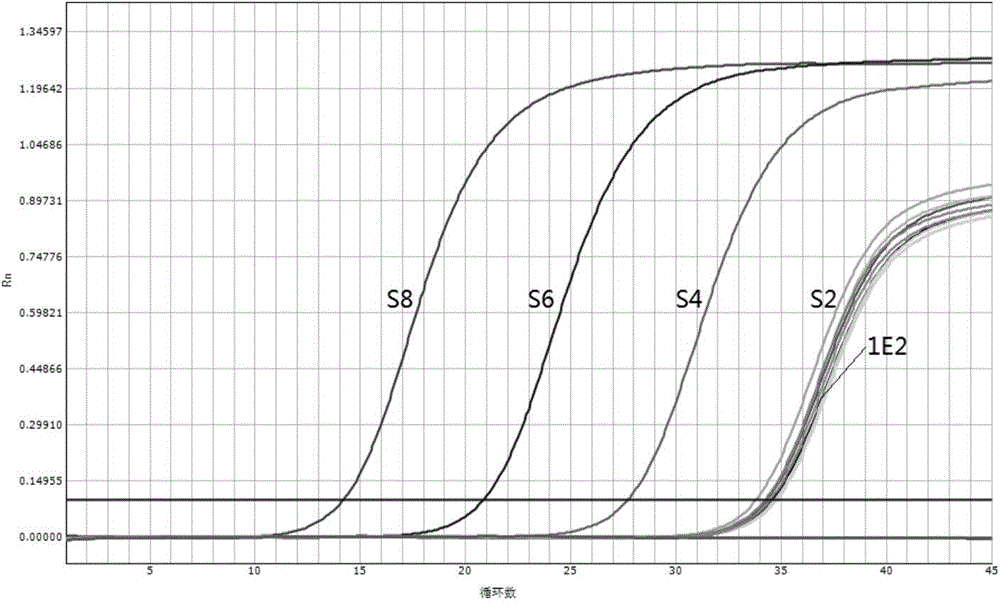
[0075] Embodiment 2 Application of the rinsing solution of the present invention in the quantitative detection of hepatitis C virus (HCV) nucleic acid
[0076]In this embodiment, the rinsing solution provided by the present invention is used to replace the rinsing solution in the commercially available HCV nucleic acid quantitative reagent, and the drying step of the rinsing solution is omitted in the nucleic acid extraction process to carry out quantitative detection on the HCV international standard. Judging whether the rinsing solution provided by the present invention is feasible based on two aspects of sensitivity and sensitivity, the specific research materials are as follows:
[0077] (1) "Hepatitis C Virus Nucleic Acid Detection Kit (Fluorescence Quantitative PCR Method)" produced by Shanghai Haoyuan Biotechnology Co., Ltd. (Medical Device Registration Certificate Number: National Food and Drug Administration (Zhun) No. 2011 No. 3400554); This product contains nucleic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com