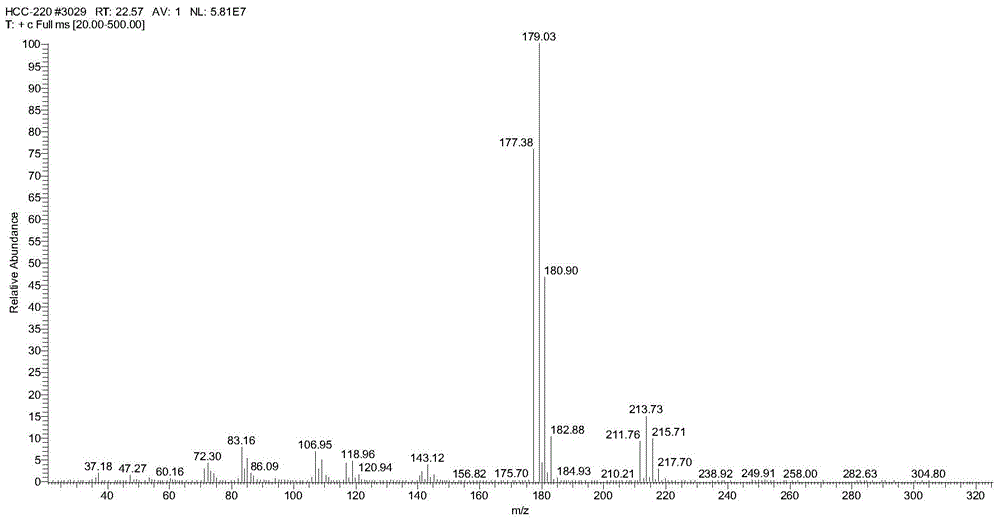
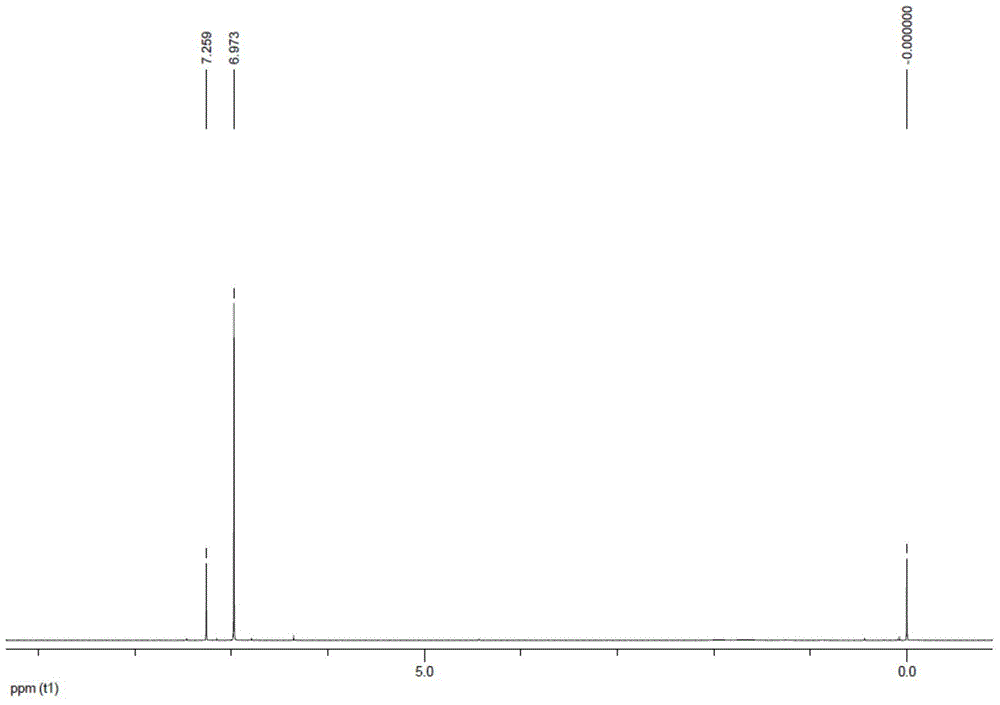
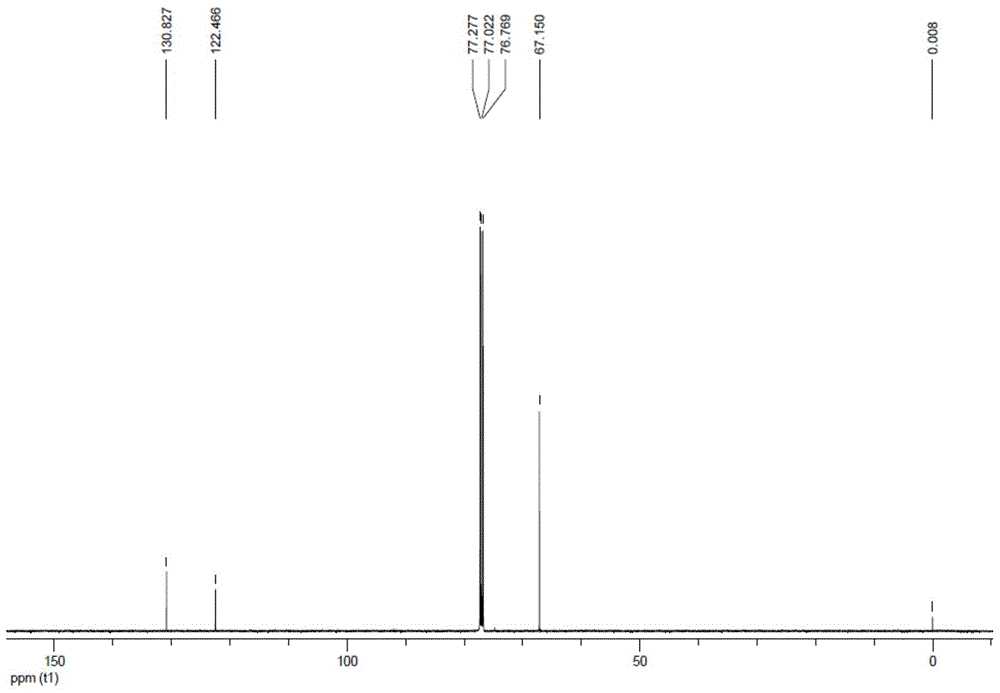
Preparation method of trans-1, 2-dichloro-3, 3, 3-trifluoropropene
A technology of trifluoropropene and pentachloropropene is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., and can solve the problems of low selectivity, unclear stereo configuration, low reaction conversion rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Catalyst preparation: according to a certain proportion, weigh Al(NO 3 ) 2 9H 2 O, Mg(NO 3 ) 2 ·6H 2 O and Cr(NO 3 ) 2 9H 2O, dissolved in deionized water, under the condition of constant stirring, dropwise add ammonia water with a mass fraction of 10%, adjust the pH control to about 7-9, about 5h, centrifuge after washing, and then dry at 120°C , then calcined at 200°C for 1 hour, raised to 320°C at 5°C / min, fired for 2 hours, raised to 400°C at 10°C / min, fired for 4 hours, and finally activated by hydrogen fluoride at 200°C-380°C.
[0032] In a fixed-bed tubular reactor with an internal diameter of 38 mm, 50 ml of the above-mentioned Mg-Al-Cr composite catalyst was loaded, wherein the molar ratio of Mg, Al, and Cr was 2:1:7, and the catalyst was dried at 200 ℃, N 2 and 1,1,1,2,2,3-hexachloropropane (HCC-230ab), react under atmospheric pressure (normal pressure), the molar ratio of the two is 5:1, the contact time is 5 seconds, the reaction product After wash...
Embodiment 2~5
[0038] Embodiment 2~5 prepares 1,1,2,3,3-pentachloropropene according to the same preparation method in embodiment 1, difference is that the reaction temperature in embodiment 1 is 200 ℃, and embodiment 2~5 The reaction temperatures were 120°C, 150°C, 240°C, and 280°C in sequence, and the results are shown in Table 1.
[0039] Table 1
[0040]
[0041]
Embodiment 6~8
[0043] Examples 6-8 Prepare 1,1,2,3,3-pentachloropropene according to the same method as in Example 1, except that the molar ratio of Mg, Al, and Cr in the catalyst in Example 1 is 2:1 : 7, while in Examples 6-8, the molar ratios of the three are 1: 1: 8, 2: 2: 6, 2: 3: 5, and the reaction results are shown in Table 2.
[0044] Table 2
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com