Process method for catalytic synthesis of 1,1,1,3,3,3-hexafluoro isopropyl methyl ether
A process method and technology of hexafluoroisopropanol, applied in catalytic synthesis 1, can solve problems such as low process yield and purity, and achieve the effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
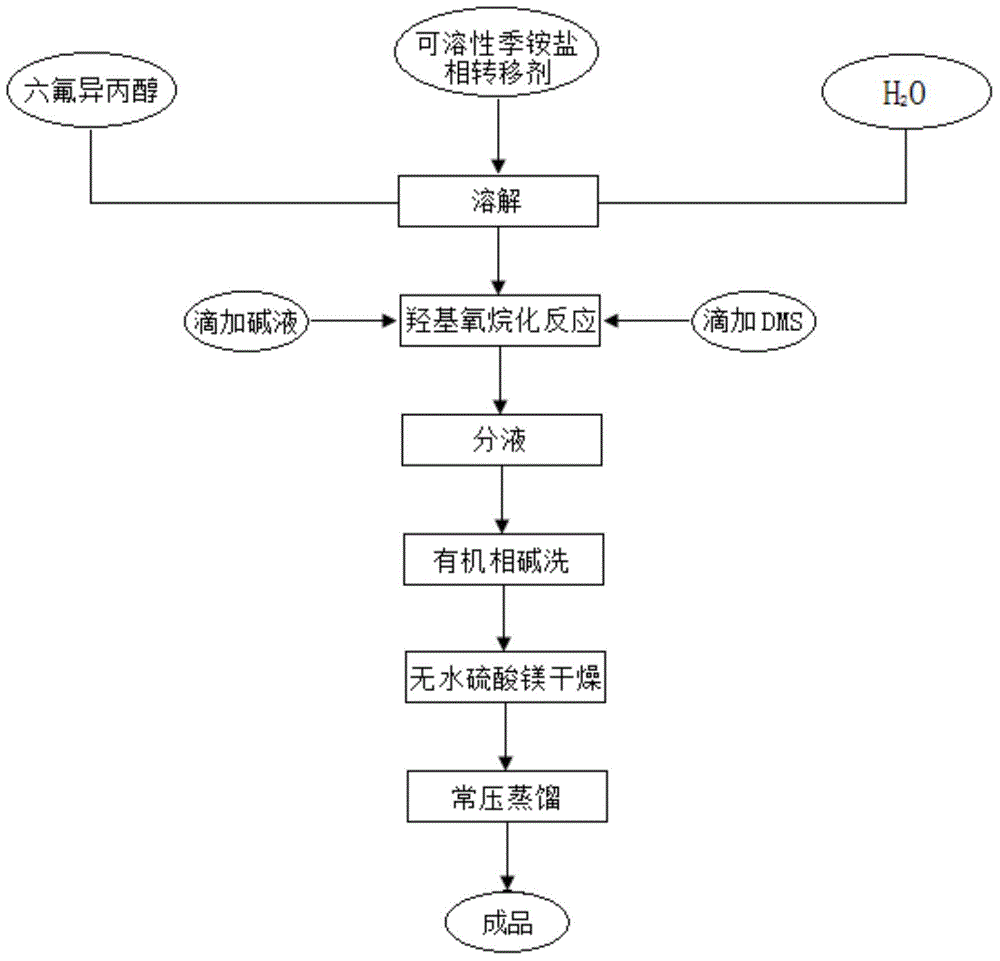
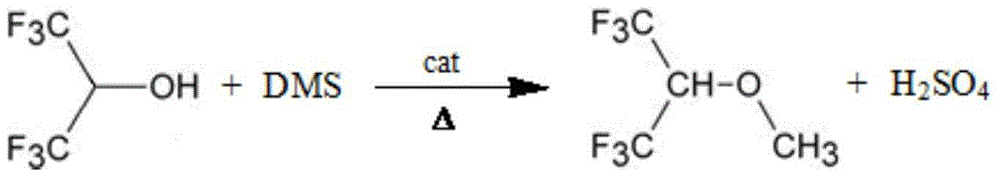
[0022] In a 250 ml four-necked flask equipped with reflux condenser, stirrer, constant pressure titration funnel, and thermometer, add quantitative water, 0.02mol tetrabutylammonium bromide, 1mol hexafluoroisopropanol, and stir to make phase transfer agent IV Butylammonium bromide is completely dissolved. Lower the temperature, slowly add lye solution dropwise, react for 1 hour, keep the temperature constant, drop 1.2 mol of dimethyl sulfate, adjust the temperature to 25-30°C, and react for 4 hours. Add lye and dimethyl sulfate, and continue the reaction for 3 hours. After the reaction is completed, the aqueous layer is removed, the organic phase is washed with lye, anhydrous magnesium sulfate is added to dry, and the 51-52°C fraction is collected by atmospheric distillation. The purity is 99.87% determined by FID.
[0023] The above process can be summarized as specific steps under the premise of the basic preparation process, that is, in step (1), 0.02 mol of tetrabutylammoniu...
Embodiment 2
[0025] In a 250ml four-necked flask equipped with reflux condenser, stirrer, constant pressure titration funnel and thermometer, add quantitative water, 0.01mol tetrabutylammonium bromide, 1mol hexafluoroisopropanol, stir to make phase transfer agent IV Butylammonium bromide is completely dissolved. Lower the temperature, slowly add lye solution dropwise, react for 1 hour, keep the temperature constant, drop 1.4 mol of dimethyl sulfate, adjust the temperature to 25-30°C, and react for 3 hours. Add lye and dimethyl sulfate, and continue the reaction for 1 hour. After the reaction is completed, the aqueous layer is removed, the organic phase is washed with lye, dried by adding anhydrous magnesium sulfate, and distilled under normal pressure to collect the fraction at 51-52°C. The purity is determined to be 99.5%.
[0026] The above process can be summarized as the specific steps under the premise of the basic preparation process, that is, in step (1), 0.01 mol of tetrabutylammoniu...
Embodiment 3
[0028] In a 250 ml four-necked flask equipped with reflux condenser, stirrer, constant pressure titration funnel and thermometer, add quantitative water, 0.04mol tetrabutylammonium bromide, 1mol hexafluoroisopropanol, and stir to make phase transfer agent IV Butylammonium bromide is completely dissolved. Lower the temperature, slowly add lye solution dropwise, react for 2 hours, keep the temperature constant, drop 1.3 mol of dimethyl sulfate, adjust the temperature to 25-30°C, and react for 2 hours. Add lye and dimethyl sulfate, and continue the reaction for 2 hours. After the reaction is completed, the aqueous layer is removed, the organic phase is washed with lye, anhydrous magnesium sulfate is added to dry, and the 51-52°C fraction is collected by atmospheric distillation. The purity is determined to be 99.6%.
[0029] The above process can be summarized as a specific step under the premise of the basic preparation process, that is, in step (1), 0.04 mol of tetrabutylammonium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com