Sodium alginate compound immobilized microbial agent as well as preparation method and application thereof
A technology of sodium alginate and bacterial agents, applied in biochemical equipment and methods, chemical instruments and methods, fixed on/in organic carriers, etc., can solve the problem of insufficient degradation efficiency of quinclorac and achieve applicable Wide range of media types, enhanced buffering capacity, and wide temperature adaptation range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The sodium alginate composite immobilized bacterial agent of the present invention comprises bacterial strains, sodium alginate, calcium chloride, an adsorption carrier composed of corncobs, bamboo charcoal and oil dryness, and an inorganic salt liquid medium.
[0042]In this embodiment, the bacterial strain is Pseudomonas stutzeri purchased from the Guangdong Provincial Microbial Culture Collection Center, and its number is GIM1.446. Since the above-mentioned Pseudomonas stutzeri is used, the present invention preferably uses The inorganic salt liquid medium for bacterial strain growth, the inorganic salt liquid medium is referred to as MSM, the composition of the MSM includes: FeCl 3 0.15g, MnCl 2 0.15g, NaNO 3 0.5g, K 2 HPO 4 1.70g, CaCl 2 ﹒ 2H 2 O0.01g, KH 2 PO 4 1.50g, FeSO 4 ﹒ 7H 2 O0.04g, MgSO 4 ﹒ 7H 2 O0.2g, (NH4) 2 SO 4 1.0g, pH﹦7, H 2 O1000ml.
[0043] The concrete preparation method of immobilized bacterial agent of the present invention is ...
Embodiment 2
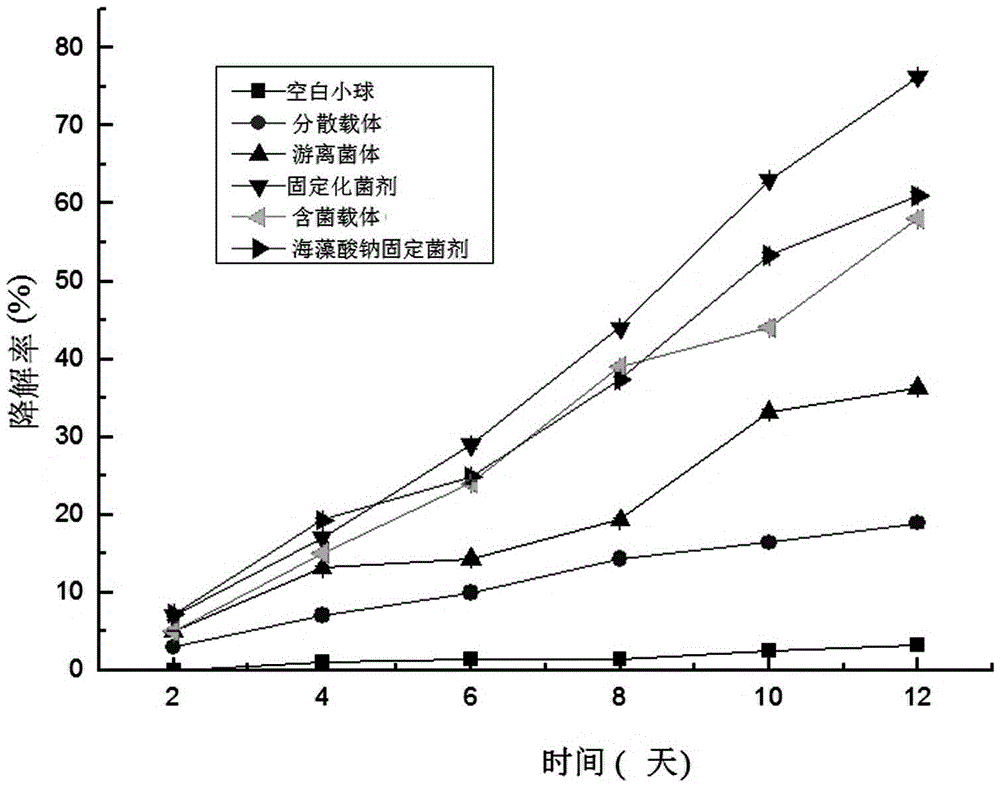
[0061] In this example, the immobilized bacterial agent prepared in Example ③ composition ratio in Example 1 is used, and the inoculum amount is 10%. Then the effects of blank pellets, dispersed carriers, free bacteria, bacteria-containing carriers, and sodium alginate-fixed bacteria agents on the removal of quinclorac from soil samples were investigated. The difference between the above dosage form and the immobilized bacterial agent is only that: the blank pellet does not contain the bacterial mother liquid; the dispersed carrier refers to the adsorption carrier in the immobilized bacterial agent; the bacterial carrier refers to the adsorption carrier with the bacterial mother liquid; Sodium acid fixative does not contain adsorption carrier.
[0062] The specific detection method is: remove the gravel and pass through a 3mm sieve to measure the content of quinclorac, and add quinclorac after sieving to make the content reach 1000mg.kg -1 After stabilizing for 5 days, measur...
Embodiment 3
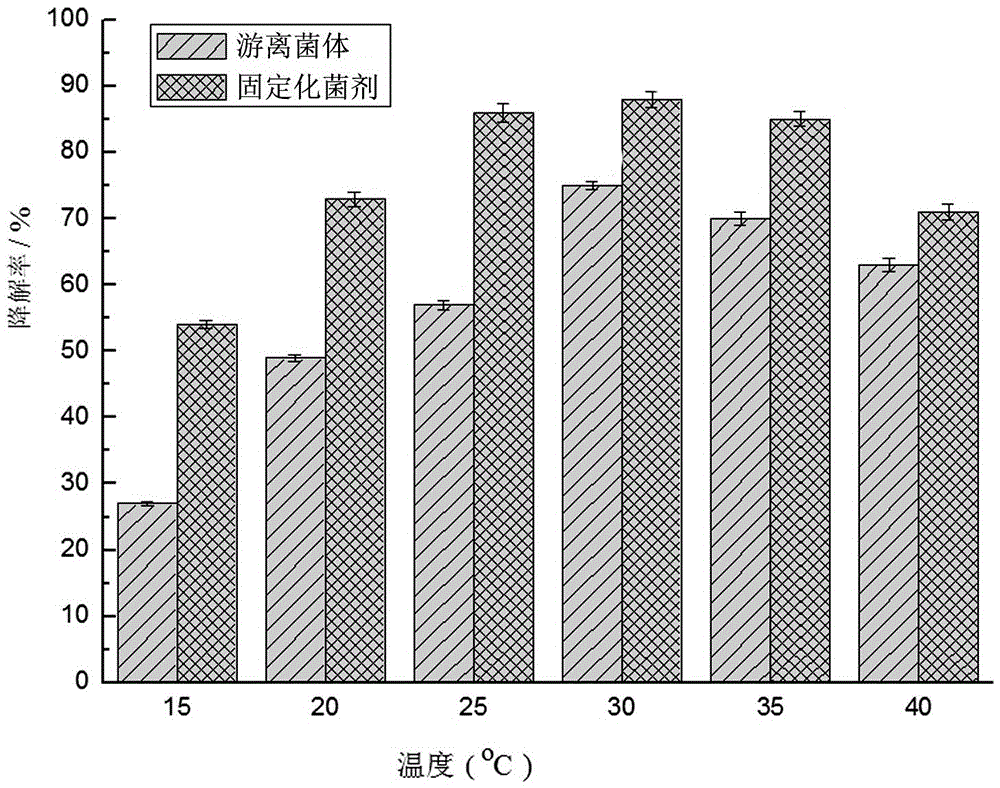
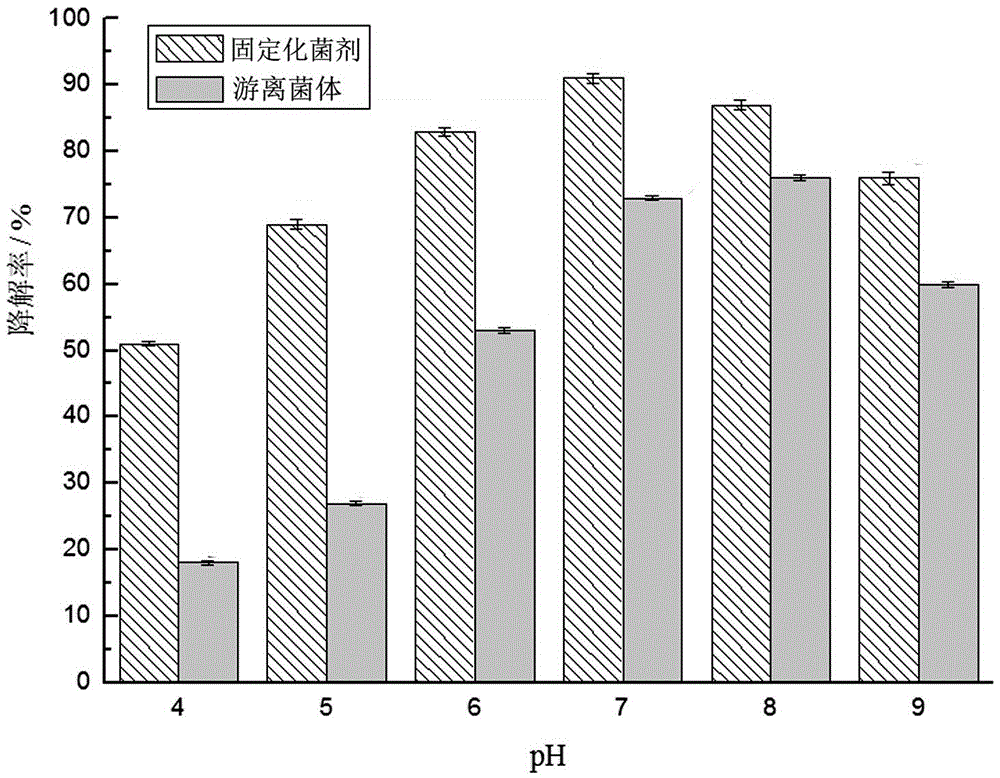
[0067] This embodiment adopts the immobilized bacterial agent made in the composition ratio of example ③ in Example 1, and uses it to detect the removal rate of quinclorac at different temperatures and different initial pH concentrations. The detection method is: 10% of the immobilized bacteria Bacteria and 3% free bacteria were added to MSM containing 800mg / L quinclorac respectively, cultured on a shaker at 140r / min, and the content of quinclorac was measured at regular intervals. The effects of temperature 15℃, 20℃, 25℃, 30℃, 35℃ and initial pH of 4, 5, 6, 7, 8, 9 on the degradation effect were investigated respectively. When examining the influence of temperature, the initial pH is 7, and when examining the initial pH, the temperature is 30°C, and the test results are as follows figure 2 and image 3 shown.
[0068] pass figure 2 It can be seen that:
[0069] The degradation rate of the immobilized bacterial agent was the highest when the temperature was 30℃, reaching...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com