Application of mesenchymal stem cell-derived exosome in preparing drug or preparation for treating preeclampsia
A kind of stem cell, preeclampsia technology, applied in the application field of exosomes in the treatment of preeclampsia, to achieve the effect of alleviating symptoms, reducing inflammatory response, and a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Isolation and culture of human umbilical cord mesenchymal stem cells (MSCs) (the reagents and consumables used in the laboratory are all aseptically processed)
[0023] With the informed consent of the puerpera, the umbilical cords of the cesarean section fetuses of normal full-term pregnancy were collected. Before the umbilical cord is collected, the puerpera needs to undergo strict pathogen testing, including Treponema pallidum, HIV, cytomegalovirus, hepatitis B virus, hepatitis C virus, syphilis and mycoplasma, and use it after confirming safety.
[0024] Cut the umbilical cord near the placenta to 20-30 cm, store it in pre-cooled sterile phosphate buffered saline (PBS) at 4°C, and use it within 4 hours. In the ultra-clean workbench, the umbilical cord was cut into small sections of about 5 cm, and the residual blood on the surface of the umbilical cord was washed with PBS; DMEM / F12 medium (purchased from Gibco, the same below), cut into 2mm with surgical ...
Embodiment 2
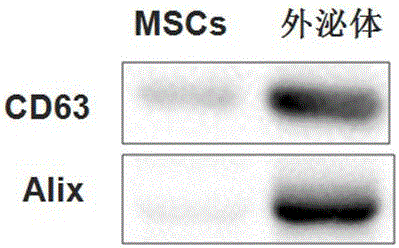
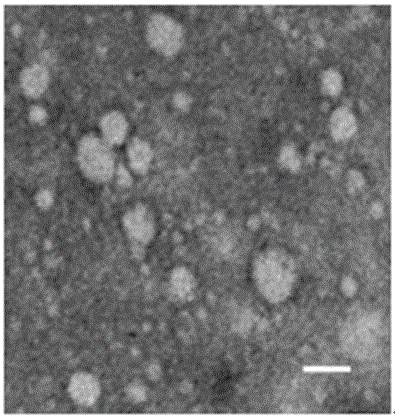
[0026] Example 2 Extraction and Identification of MSCs-derived Exosomes
[0027] (1) Extraction of MSCs-derived exosomes
[0028] Collect the MSCs culture supernatant of passages 3-7 into 500ml sterile centrifuge bottles or 50ml polypropylene centrifuge tubes (purchased from Beckman), and centrifuge at 4°C and 2000g for 10 minutes to remove dead cells and large debris. Carefully transfer the supernatant to a new sterile centrifuge tube and centrifuge at 10,000g for 30 minutes at 4°C to remove organelles and small particles. Carefully transfer the supernatant to a sterile ultracentrifuge tube, and centrifuge at 110,000g (Beckman ultracentrifuge) at 4°C for 70 minutes, discard the supernatant carefully, wash once with normal saline for injection, and store at 4°C, 110,000g ultracentrifugation for 70 minutes, the obtained pellet is exosomes. Depending on the volume of the culture medium collected initially, a small volume of normal saline for injection was added as appropriate ...
Embodiment 3
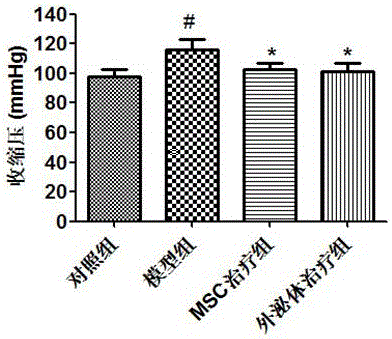
[0033] Example 3 The therapeutic effect of MSCs and their exosomes on preeclampsia (PE) mice
[0034] The C57 / B6 mice used were 8-10 weeks old and purchased from the Institute of Model Animals, Nanjing University.
[0035] (1) Low-dose lipopolysaccharide (LPS) induced mouse PE model
[0036] Mice were housed in a female-male ratio of 2:1, and vaginal plugs were checked in the morning. Those with vaginal plugs were considered to have successfully conceived, recorded as the 0.5th day of pregnancy, and so on. Pregnant mice were randomly divided into 4 groups: control group, model group, MSC treatment group and MSC-derived exosome treatment group, with 8 mice in each group. Referring to the method of Tiziana et al. (TizianaCotechinietal.Inflammationinratpregnancyinhibitsspiralarteryremodelingleadingtofetalgrowthrestrictionandfeaturesofpreeclampsia.JExpMed.2014,211(1):165-179), low-dose LPS was used to induce PE-like symptoms in mice. Model group: From day 13.5 to day 16.5, intra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com