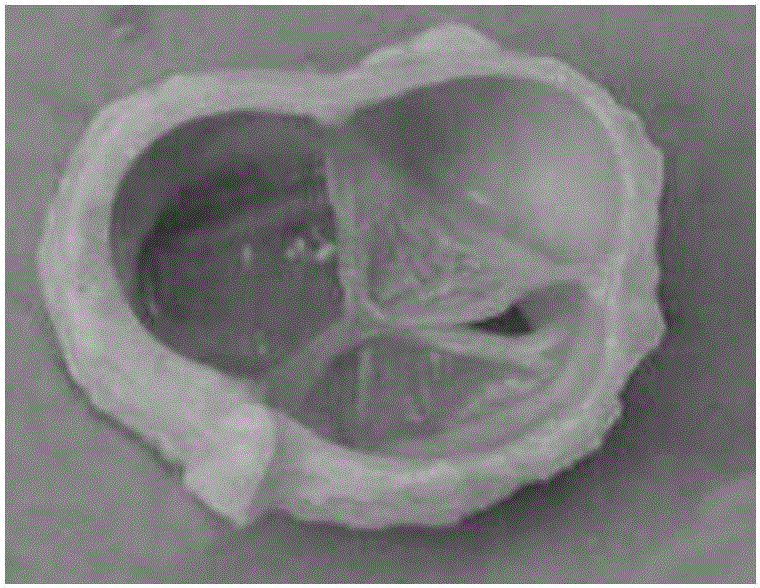
Preparing method of biological source venous valve
A venous valve, bio-derived technology, applied in the field of biomedical engineering, can solve the problems of not eliminating the root cause of varicose veins, unable to restore the venous valve, affecting the appearance of limbs, etc., achieving good biophysical properties, and not easy to acute immune rejection. , the effect of improving antithrombotic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] a. Take fresh bovine jugular vein pipes with a diameter of 0.5-1.5 cm and with valves slaughtered daily in slaughterhouses, turn them over and find the valve part, cut out a section with a length of 3-5 cm that contains a complete valve, turn it back and trim it Adipose tissue and excess connective tissue around the blood vessels were removed, and blood clots were removed by antegrade lavage with normal saline, then immersed in 0.1% brogeramine solution for disinfection for 30 minutes, and then rinsed with PBS solution for later use.
[0026] b. The material obtained in step a was treated with PBS solution containing 0.5% TritonX-100 for 48 hours, the cells were lysed, and washed with PBS solution. Then immersed in PBS solution containing 0.025% trypsin and 0.02% EDTA (0.025g trypsin, 0.02g EDTA dissolved in 100mL PBS solution) for 30min, digested cells, and washed with PBS solution. Finally, immerse in PBS solution containing 30u / ml DNaseI and 0.3mg / ml RNaseA for 24 ho...
Embodiment 2
[0031] The preparation process of the venous valve in this example is basically the same as that in Example 1, the difference is that the heparin binding process only includes one covalently bonded heparin treatment process described in step c, and the whole heparin binding process can be abbreviated as c. The obtained finished venous valve is stored in the same manner as in Example 1, that is, placed in 70-80% alcohol and sealed at room temperature.
Embodiment 3
[0033] The preparation process of the venous valve in this example is basically the same as in Example 1, the difference is that: 1. The time for cell lysis treatment, cell digestion and nucleic acid digestion treatment in step b is 36h, 60min and 36h respectively; 2. The heparin binding process only Including the ion-binding heparin treatment process described in step d once, the whole heparin binding process can be abbreviated as d. The obtained finished venous valve is placed in 70-80% alcohol and sealed and stored at room temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com