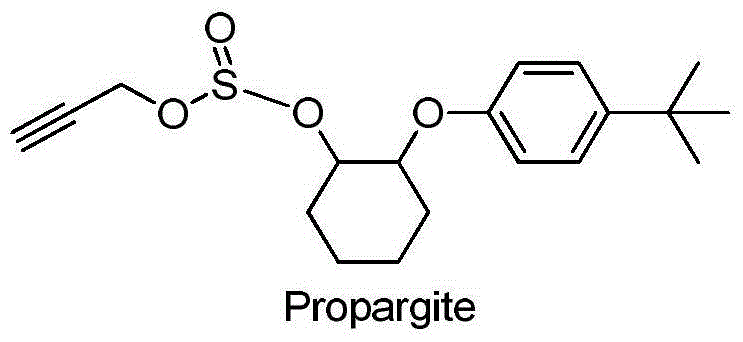
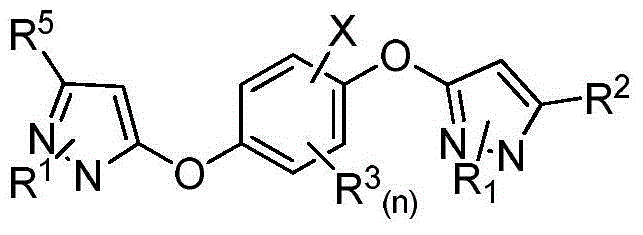
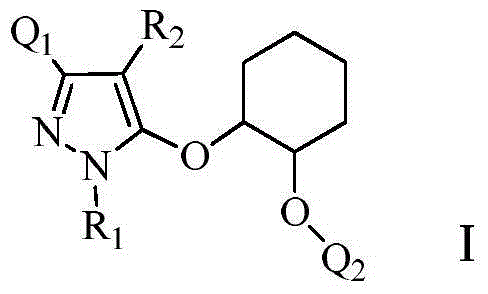
Pyrazole cyclohexanediol ether compounds and applications thereof
A technology of pyrazole cyclohexanediol ether and compound, applied in application, organic chemistry, biocide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Embodiment 1: the preparation of compound 5-1
[0130]
[0131] (1) Preparation of intermediate 3-(4-chlorophenyl)-1-methyl-5-pyrazolol
[0132] A solution of 10.60 g of methyl p-chlorobenzoylacetate in 50 ml of methanol was heated to reflux. A slight excess of methylhydrazine was added dropwise and refluxed for 3 hours. After the reaction was monitored by TLC, it was concentrated, cooled, and crystals were precipitated. Filter and rinse the crystals with a small amount of methanol. After drying, 7.5 g of crystalline 3-(4-chlorophenyl)-1-methyl-5-pyrazolol was obtained.
[0133] (2) Preparation of Compound 5-1
[0134] In a 500mL three-necked flask, add 23.05g (0.10mol) intermediate 3-(4-chlorophenyl)-1-methyl-5-pyrazolol and 10.78g (0.11mol) epoxycyclohexane, and then add 0.4 g (0.01mol) NaOH is used as a catalyst, and toluene is used as a solvent, and the reaction is carried out at 105-108° C. for 8 hours by heating. Cool to room temperature, add water, separ...
Embodiment 2
[0135] Embodiment 2: the preparation of compound 5-99
[0136]
[0137] In the 100mL flask, add 0.66g (2mmol) intermediate 2-(3-(4-chlorophenyl)-1-methyl-1H-pyrazol-5-yloxy)cyclohexanol (5-1) and 0.10g (60%, 2.4mmol) NaH, then add 20mL DMF as a solvent, react at room temperature for 30min, then add dropwise 0.29g (2.4mmol) allyl bromide solution in DMF to the bottle, then heat up to 80°C React left and right. After the reaction was monitored by TLC, after cooling to room temperature, the reaction mixture was poured into 20 mL of water, extracted with 200 mL of ethyl acetate, and the residue was separated by column chromatography to obtain 0.37 g of a colorless oil with a yield of 53.6%.
Embodiment 3
[0138] Embodiment 3: the preparation of compound 5-206
[0139]
[0140] In a 250mL flask, add 6.57g (20.0mmol) intermediate 2-(3-(4-chlorophenyl)-1-methyl-1H-pyrazol-5-yloxy)cyclohexanol and 3.03g (30.0mmol ) triethylamine, then add 100mL of dichloromethane as a solvent, add dropwise a solution of 2.71g (24mmol) of chloroacetyl chloride in dichloromethane to the bottle under stirring at room temperature, and then continue to stir the reaction at room temperature. After the reaction was monitored by TLC, the solvent was spin-dried, the mixture was poured into 50 mL of water, 200 mL of ethyl acetate was added for extraction, the organic layer was separated, washed with 10 mL of saturated brine, dried over anhydrous magnesium sulfate, and precipitated under reduced pressure. Column chromatography separated 6.89 g of brown oil, yield 85.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com