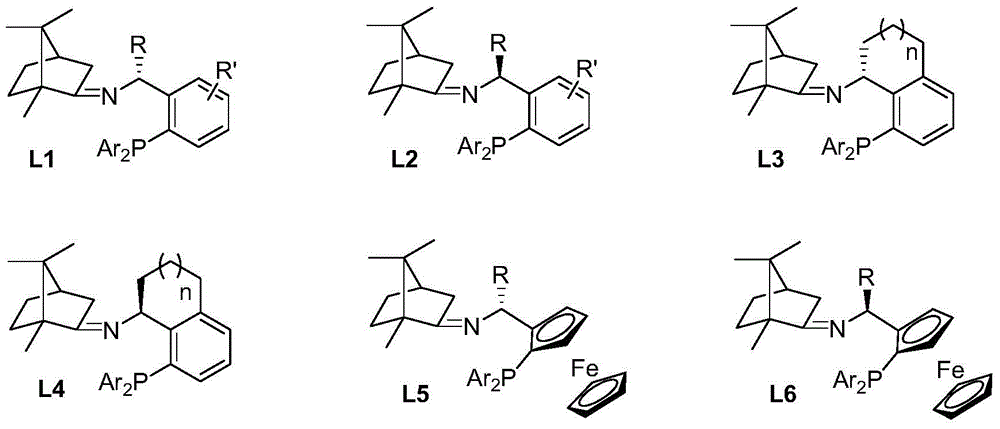
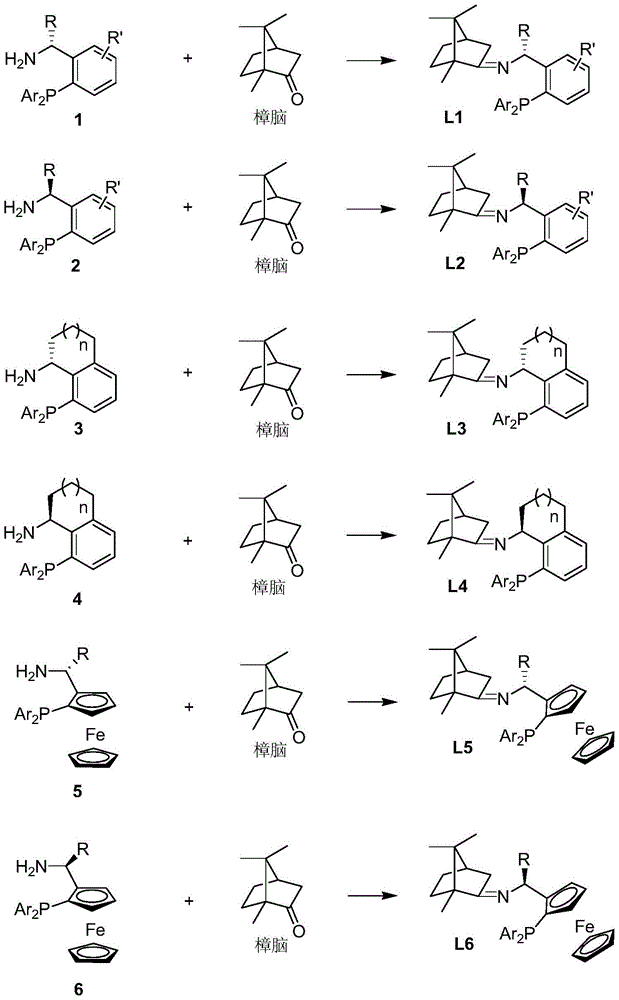
Imine ligand containing camphor
An imide phosphine and camphor technology, applied in the field of imid phosphine ligands, can solve the problems of poor enantioselectivity and yield, affecting industrial application, complex ligand synthesis, etc., achieving simple operation, easy structure modification, The effect of a simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (S)-1-(2-(diphenylphosphine)phenyl)ethyl-N-(1,7,7-trimethyl-bicyclo[2,2,1]heptane)-2-imine Synthesis.
[0029] Add p-methylbenzene to a solution of (S)-1-[2-(diphenylphosphine)phenyl]ethylamine (2.135g, 7.0mmol) and natural camphor (1.064g, 7.0mmol) in toluene (35mL) Sulphonic acid (5mg), heated under reflux for twelve hours, evaporated toluene in vacuo. The residue was extracted with ethyl acetate (3×10 mL), the organic phases were combined, and the organic phases were washed with water and saturated sodium chloride solution. After drying over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure to obtain a crude product that was further separated and purified by column chromatography to obtain a pure product (S)-1-(2-(diphenylphosphine)phenyl) as a colorless oil Ethyl-N-(1,7,7-trimethyl-bicyclo[2,2,1]heptane)-2-imine 2.551 g, yield 83%. 1HNMR (400MHz, CDCl 3 )δ7.77(dd, J=7.1,4.2Hz,1H),7.27–7.11(m,11H),6.98(td,J=7.6,1.0Hz,1H),6.72(ddd,J=...
Embodiment 2
[0031] (R)-1-(2-(diphenylphosphine)phenyl)ethyl-N-(1,7,7-trimethyl-bicyclo[2,2,1]heptane)-2-imine Synthesis.
[0032] Add benzenesulfonic acid (5mg) to (R)-1-[2-(diphenylphosphine)phenyl]ethylamine (1.525g, 5.0mmol) and natural camphor (0.760g, 5.0mmol) in toluene (25mL ) solution, heated to reflux for twelve hours, and evaporated the toluene under reduced pressure. The residue was extracted with ethyl acetate (3×10 mL), the organic phases were combined, and the organic phases were washed with water and saturated sodium chloride solution. After drying over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure to obtain the crude product, which was further separated and purified by column chromatography to obtain the pure product (R)-1-(2-(diphenylphosphine)phenyl) Ethyl-N-(1,7,7-trimethyl-bicyclo[2,2,1]heptane)-2-imine 1.823g, yield 83%. 1 HNMR (400MHz, CDCl 3 )δ7.74(dd, J=7.1,4.3Hz,1H),7.26–7.12(m,11H),7.00(td,J=7.6,1.1Hz,1H),6.76–6.73(m,1H),5.1...
Embodiment 3
[0034] (S)-1-(2-(diphenylphosphine)phenyl)propyl-N-(1,7,7-trimethyl-bicyclo[2,2,1]heptane)-2-imine Synthesis.
[0035] To a solution of (S)-1-[2-(diphenylphosphine)phenyl]propylamine (1.596 g, 5.0 mmol) and natural camphor (0.836 g, 5.5 mmol) in THF (15 mL) was added tetraethyl titanate (1.140g, 5.0mmol), heated to reflux for ten hours, and evaporated THF under reduced pressure. The residue was extracted with ethyl acetate (3×10 mL), the organic phases were combined, and the organic phases were washed with water and saturated sodium chloride solution. After drying over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure to obtain a crude product that was further separated and purified by column chromatography to obtain a pure product (S)-1-(2-(diphenylphosphine)phenyl) as a colorless oil Propyl-N-(1,7,7-trimethyl-bicyclo[2,2,1]heptane)-2-imine 1.813g, yield 80%. 1 HNMR (400MHz, CDCl 3 )δ7.66-7.64(m,1H),7.21-7.11(m,11H),6.97(t,J=7.56Hz,1H),6.77-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com