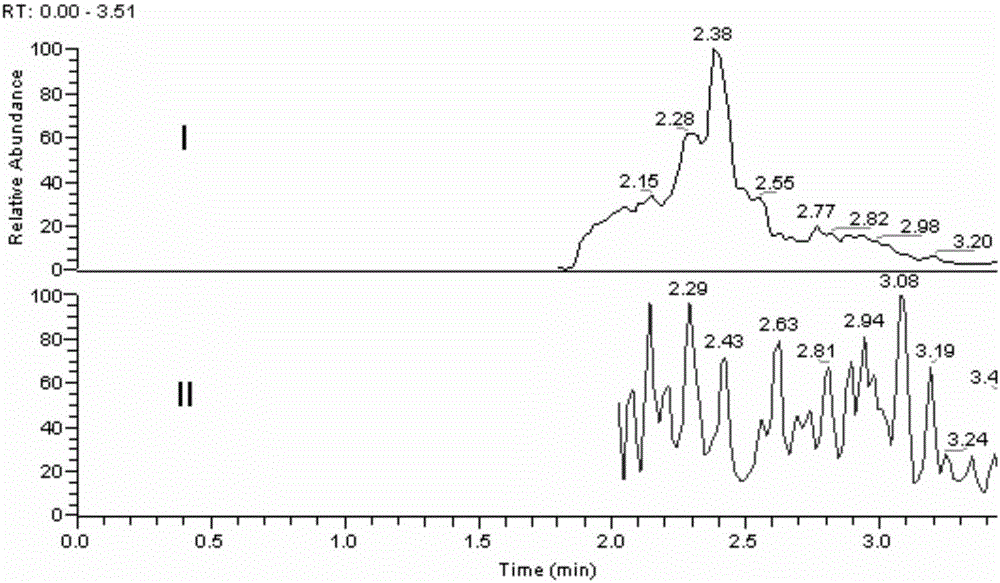
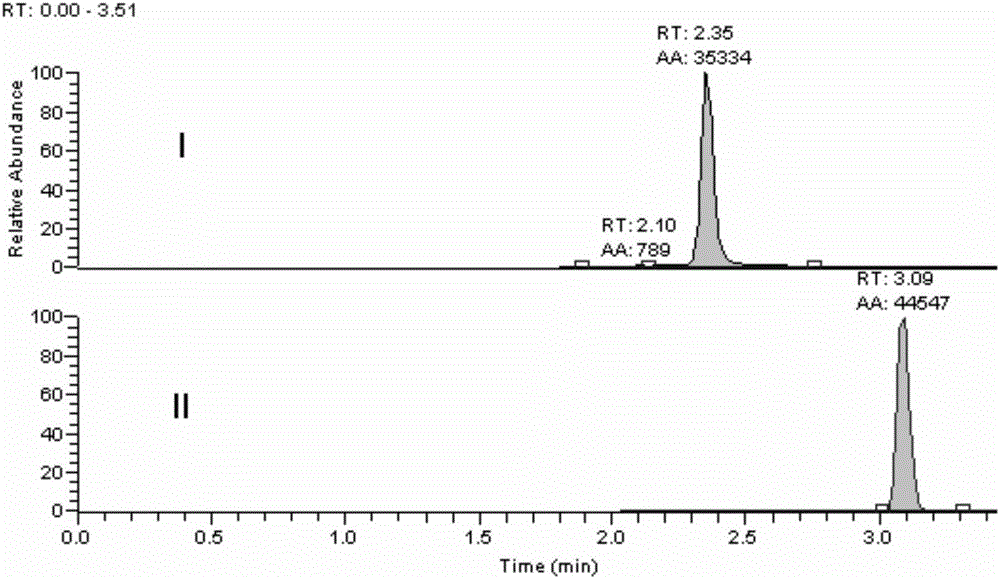
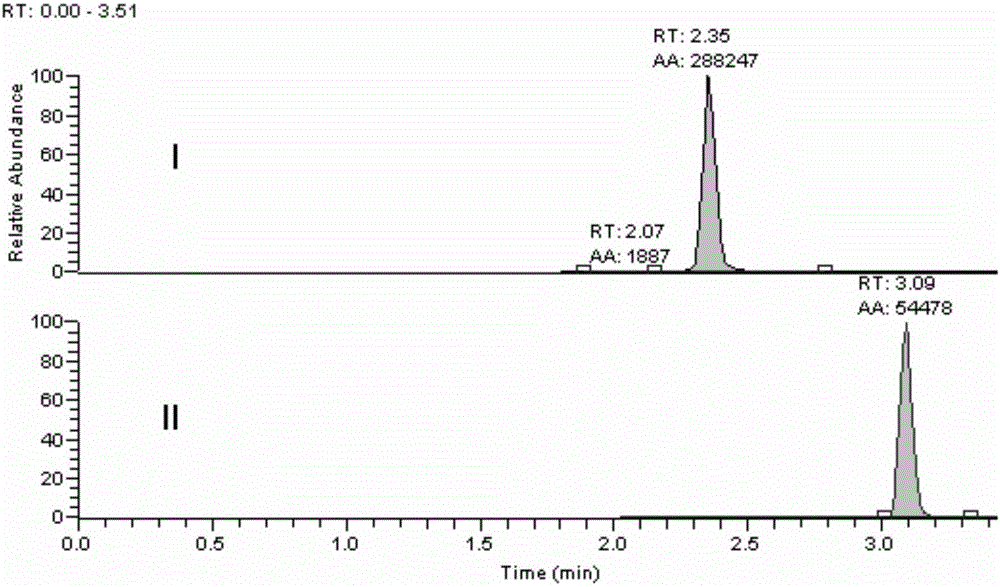
Method for determining loganin sapogenin in biological sample
A technology of strychnin, biological samples, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The methodological investigation of embodiment 1 detection method of the present invention
[0028] 1. Preparation of standard solution
[0029] (1) Preparation of standard series solutions
[0030] Accurately weigh an appropriate amount of strychnin, place it in a 10mL measuring bottle, dissolve in 0.1% formic acid water and dilute to the mark to obtain a standard stock solution with a concentration of 1.0mg / L. Precisely measure an appropriate amount of standard stock solution, dilute it with 0.1% formic acid water gradient, and prepare concentrations of 80.0, 40.0, 8.00, 2.00, 0.400, 0.0800, 0.0400 μg·mL -1 standard series solutions.
[0031] (2) Preparation of internal standard solution
[0032] Accurately weigh an appropriate amount of Dinghuan Astragalol reference substance, place it in a 10mL measuring bottle, dissolve it in methanol and dilute to the mark to obtain a concentration of 1.0mg·mL -1 stock solution. Precisely measure 0.1mL of the stock solution, ...
Embodiment 2
[0057] Example 2 Strychnin Solution Rat Pharmacokinetics Pre-test Test Method
[0058] Take 8 SD rats with a body weight of about 300g and divide them into 2 groups, 4 rats in each group. One group is intravenously injected with strychnin solution at a dose of 20 mg / kg, and the other group is given strychnin solution by gavage , the dose is 20mg / kg. On the day of administration, the body weight was weighed, and the dosage and corresponding volume were accurately calculated according to the body weight. Rats in each group were given blank blood from the subclavian vein before administration. 0.2ml of blood was collected at 0.0167, 0.0833, 0.167, 0.333, 0.5, 0.75, 1, 1.5, 2, 3, and 4 hours after intravenous administration; , 0.2ml of blood was collected at 2, 3, and 4 hours. Immediately after the blood was taken out, it was mixed with an equal volume of 0.3% formic acid saline, centrifuged at 4000rpm for 10min, and the upper layer of clarified plasma was taken, and quickly sto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com